Rheumatology treats musculoskeletal and autoimmune diseases, including arthritis, lupus, gout, and vasculitis.
Send us all your questions or requests, and our expert team will assist you.
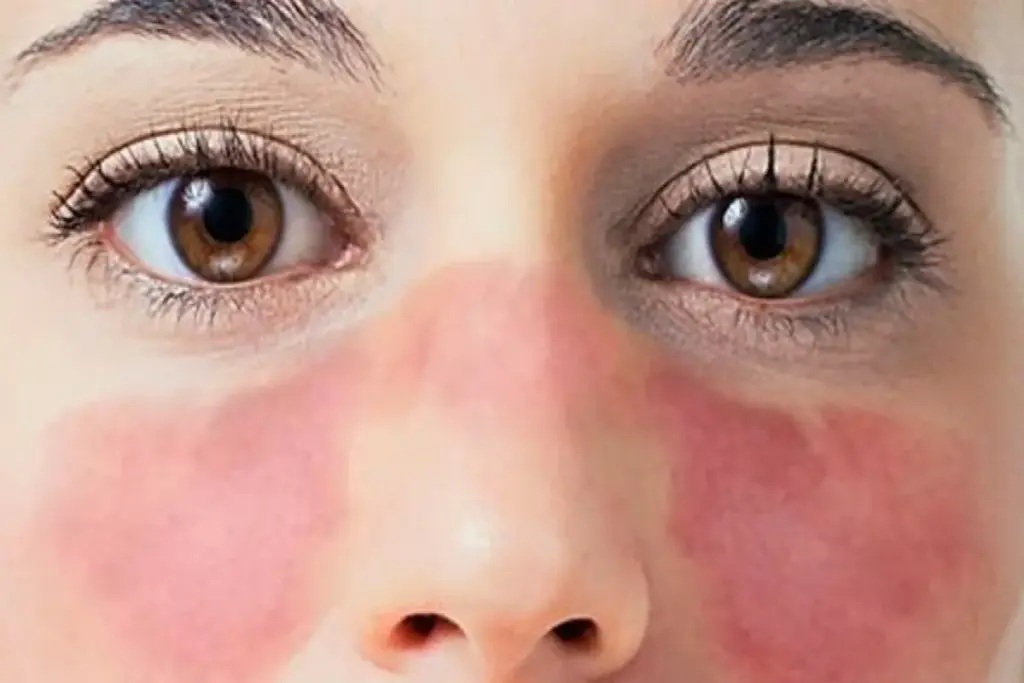
Systemic Lupus Erythematosus, commonly referred to as lupus, represents one of the most complex and multifaceted autoimmune disorders within the spectrum of clinical rheumatology. It is fundamentally a disease characterized by a loss of immune tolerance, wherein the body’s defense mechanisms fail to distinguish between foreign pathogens and its own cellular constituents. This biological error leads to the production of autoantibodies that target nuclear material, resulting in widespread inflammation and tissue damage. Unlike organ-specific autoimmune diseases that target a single system, lupus is distinct in its potential to affect virtually every organ system in the body, including the skin, joints, kidneys, brain, heart, and lungs.
The pathogenesis of lupus involves a sophisticated interplay between genetic susceptibility, hormonal factors, and environmental triggers. At the cellular level, the disease is driven by hyperactivity of B cells, the immune cells responsible for antibody production. In a healthy immune system, B-cells that react to self-antigens are eliminated or silenced through a process known as tolerance. In lupus, this checkpoint regulation fails. Consequently, B cells proliferate and differentiate into plasma cells that secrete high levels of autoantibodies, particularly antinuclear antibodies. These antibodies bind to circulating antigens to form immune complexes.
These immune complexes are the primary architects of tissue injury in lupus. They circulate in the bloodstream and deposit in the delicate capillary networks of various organs. This deposition triggers the complement cascade, a part of the immune system that enhances the ability of antibodies and phagocytic cells to clear microbes and damaged cells. However, in the context of lupus, complement activation recruits inflammatory cells, such as neutrophils and macrophages, to the site of deposition. These cells release cytotoxic enzymes and reactive oxygen species, causing collateral damage to the surrounding tissue. This cycle of immune complex deposition, inflammation, and tissue injury defines the core pathology of lupus.

While Systemic Lupus Erythematosus is the most common and severe form, the medical definition encompasses a spectrum of related conditions that share similar immunologic features but manifest differently. Understanding these distinctions is critical for accurate prognosis and management.
Discoid Lupus Erythematosus is a form of the disease primarily limited to the skin. It is characterized by coin-shaped lesions that can lead to scarring and permanent hair loss if they occur on the scalp. While a small percentage of patients with discoid lupus may progress to the systemic form, the majority experience pathology confined to the integumentary system. This variant highlights the heterogeneity of the disease, suggesting that specific tissue environments may harbor unique immunological niches that permit localized autoimmune activity without systemic spread.
Drug-Induced Lupus is a reversible form of the condition precipitated by the long-term use of certain medications. These drugs can alter the epigenetic structure of DNA or interact with immune cells to temporarily break tolerance. The clinical presentation often mimics systemic lupus, with symptoms including joint pain, pleurisy, and a rash. However, a defining feature is the resolution of symptoms upon discontinuation of the offending agent. This variant provides valuable insights into the environmental triggers of autoimmunity, demonstrating how external chemical agents can manipulate the immune system.
Neonatal Lupus is a rare condition affecting infants born to mothers with specific autoantibodies, namely anti-Ro and anti-La. These antibodies can cross the placenta and affect the developing fetus. The most serious manifestation is congenital heart block, a permanent condition requiring pacemaker implantation. Cutaneous manifestations in the neonate typically resolve as the maternal antibodies are cleared from the infant’s circulation, illustrating the concept of passive autoimmunity.

In the evolving landscape of modern medicine, lupus is increasingly viewed not just as a condition of chronic inflammation but as a failure of homeostatic regulation and tissue repair. The chronic inflammation associated with lupus often overwhelms the body’s natural regenerative capacities. For instance, in lupus nephritis, the ongoing immune assault leads to nephron loss and the development of renal fibrosis. The regenerative perspective seeks to understand why the local stem cell populations fail to repair this damage effectively.
Research in cellular biology has identified defects in the mesenchymal stem cells of patients with lupus. These cells, which are typically responsible for modulating immune responses and supporting tissue repair, appear to be functionally impaired in patients with lupus. They exhibit reduced capacity to suppress T-cell proliferation and may undergo premature senescence or aging. This finding has profound implications, suggesting that the disease involves a systemic exhaustion of the body’s reparative machinery.
Consequently, therapeutic strategies are shifting towards restoring this regenerative potential. The concepts of resetting the immune system through hematopoietic stem cell transplantation or modulating the inflammatory environment with healthy mesenchymal stem cells are at the forefront of advanced research. This approach aims to move beyond symptom suppression towards re-establishing immune tolerance, effectively teaching the immune system to stop attacking self-tissues while preserving its ability to fight infection.
Lupus is a global health challenge with a distribution that reflects complex genetic and environmental influences. The disease exhibits a striking gender disparity, with women of childbearing age being significantly more affected than men. This female preponderance suggests a critical role for sex hormones, particularly estrogen, in modulating the immune system. Estrogen is known to enhance B-cell survival and antibody production, which may predispose genetically susceptible women to autoimmunity.
Ethnicity plays a significant role in the prevalence and severity of the disease. Epidemiological data indicate that lupus is more common and often more severe in populations of African, Asian, and Hispanic descent compared to Caucasians. These disparities are likely due to a combination of genetic polymorphisms that affect immune regulation, socioeconomic factors that influence access to care, and environmental exposures.
The burden of the disease extends beyond mortality to significant morbidity. Patients often face a lifetime of relapsing and remitting symptoms that impact physical function, mental health, and quality of life. The chronic nature of the condition necessitates a comprehensive care model that addresses not only the acute flares but also the long-term consequences of chronic inflammation, such as accelerated cardiovascular disease and osteoporosis.

A central theme in the modern understanding of lupus is the defect in the clearance of apoptotic cells. Apoptosis is the process of programmed cell death, a normal physiological mechanism that removes old or damaged cells. In a healthy individual, these dying cells are rapidly cleared by phagocytes without eliciting an inflammatory response.
In patients with lupus, this clearance mechanism is often defective. Apoptotic cells accumulate in tissues, eventually undergoing secondary necrosis. This process releases intracellular nuclear material, which is usually hidden from the immune system, into the extracellular space. This exposed nuclear debris acts as a potent antigen, stimulating the production of anti-nuclear antibodies. This hypothesis, known as the waste disposal theory, redefines lupus as a disorder of cellular housekeeping. It suggests that enhancing the body’s ability to clear cellular debris could potentially reduce the antigenic load and dampen the autoimmune response. This insight bridges the gap between basic cellular biology and clinical pathology, offering new targets for therapeutic intervention that enhance phagocytic function.
Send us all your questions or requests, and our expert team will assist you.
The central pathology is defined by the loss of immune tolerance, leading the body to produce autoantibodies against its own nuclear material. This results in the formation of immune complexes that deposit in tissues, triggering the complement system and recruiting inflammatory cells that cause organ damage.
Lupus is considered a multifactorial disease that arises from the interaction of genetic susceptibility and environmental triggers. While genes provide the predisposition, environmental factors such as ultraviolet light, infections, or stress are often required to initiate the active disease process.
The regenerative perspective views lupus as a failure of both immune regulation and tissue repair. It highlights defects in the patient’s own mesenchymal stem cells, which fail to modulate the immune system effectively, suggesting that therapies should focus on restoring these cellular functions.
The prevalence in women is mainly attributed to hormonal differences, particularly the influence of estrogen. Estrogen can enhance B-cell activity and antibody production, creating a more favorable environment for autoimmune activity in individuals who are genetically predisposed.
Immune complexes are clusters of antibodies bound to antigens. In lupus, they are the primary mechanism of tissue injury because they become trapped in small blood vessels and organs such as the kidneys. Their presence activates the immune system’s inflammatory response, leading to the physical destruction of the tissue where they are deposited.
