We are seeing a major breakthrough in medicine. KJ Muldoon, the first baby treated with CRISPR gene-editing therapy, is a key example. He was born with a rare and dangerous condition called severe carbamoyl phosphate synthetase1 (CPS1) deficiency. At six months old, he got his first dose of the therapy at the Children’s Hospital of Philadelphia.
This treatment is a big step forward in genetic medicine. It shows a new era of precision medicine. This era is changing how we treat genetic diseases that were once thought to be untreatable.
Key Takeaways
- KJ Muldoon was the first baby to receive a customized CRISPR gene-editing therapy.
- The therapy was administered at the Children’s Hospital of Philadelphia.
- KJ was born with severe carbamoyl phosphate synthetase1 (CPS1) deficiency.
- The treatment has shown significant promise in addressing previously untreatable genetic diseases.
- KJ has tolerated the therapy with no serious side effects reported.
The Groundbreaking Case of KJ Muldoon
KJ Muldoon’s treatment with CRISPR gene editing has brought hope to families with rare genetic conditions. He was diagnosed with CPS1 deficiency, a rare metabolic disorder. This affects 1 in 1.3 million newborns and makes it hard to break down ammonia, which can be deadly.

A Rare and Life-Threatening Condition
CPS1 deficiency is a severe genetic disorder that needs quick and effective treatment. KJ’s condition was caught early, and his team found that usual treatments didn’t work well. This disorder not only harms his health but also affects his development.
The Decision to Attempt Gene Editing
KJ’s medical team and family thought long and hard before trying gene editing with CRISPR-Cas9. They hoped to fix the genetic issue causing KJ’s condition. The treatment involved base editing to fix the Q335X mutation in KJ’s DNA. He got three doses of the therapy in February, March, and April 2025.
Using gene editing in KJ’s case is a big step forward in treating rare genetic disorders. It aims to lessen the effects of CPS1 deficiency and improve KJ’s quality of life.
We will dive deeper into KJ’s treatment and the science behind the gene editing technology in the next sections.
Understanding CPS1 Deficiency and Its Genetic Basis
It’s key to know the genetic reasons behind CPS1 deficiency to find good treatments. This condition stops the liver from breaking down protein byproducts. This buildup leads to too much ammonia, which is harmful.
The Role of Carbamoyl Phosphate Synthetase 1
Carbamoyl phosphate synthetase 1 (CPS1) is a vital enzyme in the urea cycle. It helps remove ammonia, a harmful byproduct of protein breakdown, from the body. The liver is where most of this cycle happens, with CPS1 starting it off by turning ammonia into carbamoyl phosphate.
This process is essential to avoid ammonia buildup. Without enough CPS1, the body can’t detoxify ammonia well. This can cause hyperammonemia, a dangerous condition with high ammonia levels in the blood.
The Q335X Mutation
The Q335X mutation affects the CPS1 enzyme’s function. It causes CPS1 to not work right, leading to CPS1 deficiency. This mutation is one of several that can cause this condition, but it’s a big deal in cases like KJ Muldoon’s.
Gene editing, like CRISPR-Cas9, might help treat CPS1 deficiency. It aims to fix the genetic problem by making CPS1 work right again. This could solve the root cause of the condition.
Key aspects of CPS1 deficiency and its genetic basis include:
- The critical role of CPS1 in the urea cycle
- The impact of genetic mutations like Q335X on CPS1 function
- The promise of gene editing to fix these mutations
Knowing the genetic cause of CPS1 deficiency helps in finding treatments. Gene editing, as seen in KJ Muldoon’s case, is a big step forward in treating genetic diseases.
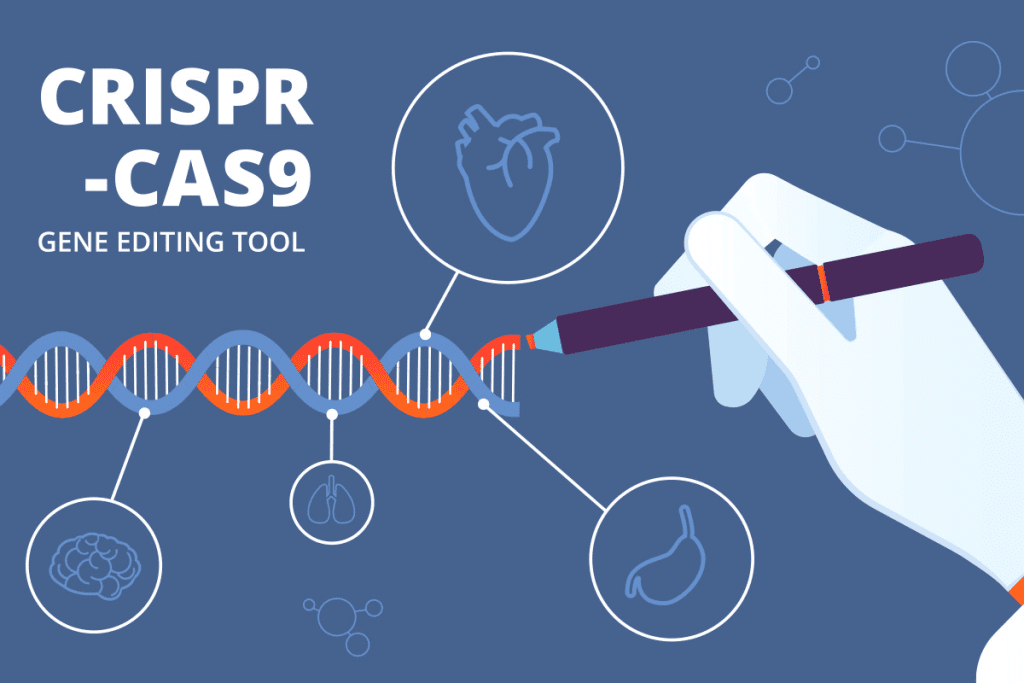
The Science of CRISPR Gene Editing
CRISPR technology is leading the way in genetic medicine. It allows for precise changes to the human genome. This is changing how we treat genetic diseases, thanks to CRISPR-Cas9 gene editing.
How CRISPR-Cas9 Technology Works
CRISPR-Cas9 is a groundbreaking tool for gene editing. It makes precise cuts in DNA at specific spots. This is done by guiding the Cas9 enzyme to the right spot with a small RNA molecule called a guide RNA.
After the DNA is cut, the cell fixes it. This can correct mutations that cause diseases.
The process involves several key steps:
- Designing a guide RNA that matches the target DNA sequence.
- Delivering the guide RNA and Cas9 enzyme to the cells.
- The Cas9 enzyme cuts the DNA at the targeted location.
- The cell repairs the cut DNA, and in doing so, the disease-causing mutation can be corrected.
Base Editing: A Refined Approach
Base editing is an improvement over CRISPR-Cas9. It changes one DNA base to another without breaking the genome. This is great for fixing point mutations, like the Q335X mutation in KJ’s case.
The benefits of base editing include:
- Precision: It fixes specific mutations without touching other parts of the genome.
- Safety: It avoids double-stranded breaks, reducing off-target effects.
- Efficiency: It can be more effective than traditional CRISPR-Cas9 editing in some cases.
Base editing was used in KJ’s treatment to fix the Q335X mutation in the CPS1 gene. This offers a precise and potentially safer way to treat genetic diseases.
As we keep improving CRISPR gene editing, we’re getting closer to making genetic medicine a reality for many diseases. The advancements in CRISPR-Cas9 and base editing are opening up new treatment options that were once unthinkable.
KJ’s Treatment Protocol and Timeline
KJ’s journey started with a detailed diagnosis and assessment. This led to a groundbreaking gene editing therapy. The first step was key to understanding his condition and finding the best treatment.
Initial Diagnosis and Assessment
Genetic testing confirmed KJ had CPS1 deficiency. It found the Q335X mutation causing his condition. This phase was important for preparing for the gene editing treatment.

The Three-Dose Treatment Regimen
In February, March, and April 2025, KJ got three doses of gene-editing therapy. The treatment used base editing to fix the Q335X mutation in his DNA. This three-dose plan was designed for safe and effective treatment.
Base editing technology was a big step forward in gene editing. It aimed to fix the genetic mutation and improve KJ’s life quality.
Immediate Post-Treatment Monitoring
After each dose, KJ was closely monitored. The team watched for any side effects or complications. This careful watch was key to the treatment’s success.
The medical team was always ready to face any challenges. Their hard work and knowledge were vital for KJ’s recovery.
Remarkable Results and Recovery
KJ, the first baby cured by CRISPR, has shown amazing results. His physical and developmental progress has improved a lot. This marks a new chapter in treating genetic disorders.
Physical and Developmental Improvements
KJ has grown strong and can now sit up independently. This is a big deal for a child with CPS1 deficiency. It shows a big improvement in his health and development.
Also, KJ can handle more dietary protein and needs less medication. These changes make his life better and show how well the gene editing therapy works.
Comparison to Expected Disease Progression
KJ’s recovery is truly remarkable compared to what’s expected for CPS1 deficiency. Kids with this condition usually face big delays and health issues. But KJ’s progress is significantly better than expected. This shows CRISPR’s power in treating diseases that were thought to be incurable.
Safety Profile and Observed Effects
The CRISPR treatment has been safe, with no big side effects. We keep a close eye on KJ’s health. This way, we can quickly deal with any side effects.
KJ’s successful treatment with CRISPR opens up new ways to treat rare genetic disorders. As we watch KJ’s progress, we’re hopeful about gene editing’s future. It could greatly improve the lives of kids with severe genetic conditions.
The Six-Month Development Miracle
KJ’s treatment story is a tale of teamwork between experts. This team worked together to create a special gene editing therapy with CRISPR-Cas9 technology.
Collaborative Research Efforts
Thanks to teamwork, KJ’s therapy was developed quickly. Centers like Children’s Hospital of Philadelphia and Penn Medicine joined forces. They used their knowledge in gene editing and genetic medicine to make the therapy.
Genetic modification companies were key in this journey. They provided the latest technology and resources. This helped the team tackle the tough challenges of creating a new gene editing treatment.
Accelerated Timeline from Concept to Treatment
KJ’s therapy was developed in just six months. This fast pace was thanks to CRISPR genome editing advancements. It allowed for quick design and making of the therapy.
Also, the regulatory process was streamlined. This helped the team move fast through the approval steps. It made sure KJ got the therapy quickly, which was very important.
Regulatory Considerations and Approvals
KJ’s therapy went through strict checks to ensure it was safe and worked well. Regulatory bodies worked hand in hand with the team. This made it possible to quickly get the therapy to KJ.
KJ’s success shows the power of fast development in genetic medicine. It shows we can quickly bring new gene editing treatments to life. As we go forward, we’ll see more teamwork to keep pushing the boundaries in this field.
Historical Context of CRISPR Gene Editing in Human Treatment
CRISPR gene editing has changed genetic medicine a lot. Looking at KJ’s case, we see how far we’ve come. It’s key to know the history behind this breakthrough.
Previous Milestones in Gene Therapy
Gene therapy has grown a lot over time. It had many important steps before CRISPR. Early tries were in the 1990s. But CRISPR-Cas9 changed everything.
- The first gene therapy trial was in 1990, for SCID.
- Glybera, the first gene therapy drug in Europe, was approved in 2012.
- CRISPR-Cas9 made gene editing easier and more precise.
These steps helped make gene editing better. They led to KJ’s treatment.
Ethical Debates and Regulatory Evolution
As gene editing got better, so did the debates about it. Issues like germline editing and off-target effects were big concerns. Rules had to change to keep up.
“The ethics of gene editing are complex and multifaceted, requiring careful consideration of both the benefits and risks.”
(Baylis, 2019; National Academies of Sciences, Engineering, and Medicine, 2017).
New rules helped KJ’s treatment move fast but safely. The FDA’s guidelines were key.
Why KJ’s Case Represents a Historic Turning Point
KJ’s case is big because of CRISPR’s success. The therapy was ready in just six months. This shows gene editing can fix diseases we couldn’t before.
Key factors for this success were:
- Teamwork among scientists, doctors, and regulators.
- Improvements in CRISPR tech, like base editing.
- Quick but safe rules for new treatments.
KJ’s story is a big step for gene editing. It shows what’s possible for treating genetic diseases.
N-of-1 Genetic Therapies: Treating Ultra-Rare Disorders
KJ’s story shows the power of N-of-1 genetic therapies for ultra-rare disorders. These treatments offer hope to those with unique genetic conditions. They are made just for one person with a rare genetic disease.
Defining and Identifying N-of-1 Conditions
N-of-1 conditions are very rare, affecting just one person or family. Finding these conditions needs advanced genetic analysis and deep knowledge of the disease’s genetics.
The process involves:
- Advanced genomic sequencing to find the genetic mutation causing the condition.
- Detailed clinical evaluation to understand how the disease shows up and grows.
- Working together between doctors, geneticists, and researchers to create a treatment plan just for that person.
Economic and Ethical Considerations
Creating N-of-1 genetic therapies brings up big economic and ethical questions. Making a treatment just for one person can be very expensive. There are also worries about who can get these treatments and if it’s fair.
Key considerations include:
- The high cost of making personalized treatments and the need for new ways to pay for them.
- Debates about using gene editing for rare conditions.
- How to make sure decisions about who gets these treatments are clear and fair.
Scalability Approaches for Individualized Treatments
Even though N-of-1 genetic therapies are for one person, researchers are looking for ways to make them more available. They want to find ways to use the same technology for different rare genetic conditions.
Strategies for scalability include:
- Designing gene editing tools in a way that makes them easy to change for different mutations.
- Working together with other research groups and companies to share resources and knowledge.
- Investing in new technologies that make N-of-1 therapies cheaper and more efficient.
By improving N-of-1 genetic therapies, we’re not just treating rare disorders. We’re also opening up new paths in genetic medicine. As we keep working on these treatments, we need to think about the science, money, and ethics. We want to make sure everyone has a chance to get these life-changing treatments.
Future Implications for Genetic Medicine
KJ’s treatment with CRISPR gene editing is a big step for genetic medicine. It shows we can treat not just rare diseases but also common ones. This technology could change how we approach many health issues.
Expanding Applications to Other Genetic Disorders
The CRISPR genome editing method used in KJ’s treatment is promising. It could help cure many genetic diseases. For example, it might treat sickle cell anemia, muscular dystrophy, and Huntington’s disease.
- Precision medicine: Tailoring treatments to individual genetic profiles.
- Gene therapy: Potentially curing genetic diseases by correcting the underlying genetic cause.
- Expanded treatment options: Giving new hope to patients with previously untreatable conditions.
Accelerating Treatment Development Timelines
KJ’s treatment shows gene editing CRISPR works well in humans. This breakthrough could make new treatments faster and cheaper. It’s a big step towards better health care.
Researchers can now work faster on other genetic therapies. They can make gene editing better and safer. This will help bring new treatments to market sooner.
Long-term Monitoring and Research
As we learn genetics, CRISPR simulation and other gene editing, we must watch patients closely. We need to follow their health for a long time. This ensures the treatments are safe and work well.
- Establishing robust monitoring systems to track patient outcomes.
- Conducting ongoing research to refine gene editing techniques.
- Ensuring transparency and collaboration among researchers, clinicians, and regulatory bodies.
This careful monitoring and research will help us improve genetic medicine. We can make sure gene editing is safe and effective for everyone.
Conclusion: A New Chapter in Treating Previously Incurable Genetic Diseases
We are at a groundbreaking moment in treating genetic diseases. KJ’s gene editing therapy has shown success. This opens a new chapter in treating conditions that were once thought incurable.
CRISPR gene editing in KJ’s treatment has brought remarkable results. This success encourages genetic modification companies to explore new areas in genetic medicine. We must keep advancing gene editing, tackling both challenges and opportunities.
KJ’s treatment success gives us hope for treating other genetic disorders. The future of genetic medicine looks bright, with many possibilities. We will keep studying the long-term effects of gene editing to ensure it improves human health.
FAQ
What is CRISPR gene editing, and how does it work?
CRISPR-Cas9 is a way to change the human genome. It targets specific DNA sequences and makes precise cuts. This allows for fixing mutations that cause diseases.
What is CPS1 deficiency, and how is it caused?
CPS1 deficiency is a rare metabolic disorder. It’s caused by a mutation in the gene for carbamoyl phosphate synthetase1. This enzyme is key for the urea cycle. The Q335X mutation stops it from working right, causing ammonia buildup.
What is base editing, and how does it differ from CRISPR-Cas9?
Base editing is a new method that improves on CRISPR-Cas9. It makes precise DNA changes without breaking the DNA strands. This makes it safer and more effective.
What were the results of KJ’s gene editing treatment?
KJ’s treatment led to big improvements. He grew well and gained motor skills like sitting up on his own. There were no major side effects.
What is an N-of-1 genetic therapy, and what are its implications?
N-of-1 genetic therapies are custom treatments for rare disorders. They raise questions about cost, ethics, and how to make more treatments.
How does KJ’s case represent a historic turning point in gene editing?
KJ’s case shows gene editing’s power to cure genetic diseases. It’s a big step forward in genetic medicine, opening doors for more treatments.
What are the future implications of KJ’s case for genetic medicine?
KJ’s case could lead to new treatments for genetic disorders. It might speed up treatment development and improve ongoing research and monitoring.
What is germline editing, and how does it relate to KJ’s treatment?
Germline editing changes genes in reproductive cells, affecting future generations. KJ’s treatment was somatic, but the tech has germline implications too
How does CRISPR genome editing work, and what are its applications?
CRISPR genome editing targets DNA sequences and makes precise cuts. It can fix mutations causing diseases. It’s used for treating genetic diseases like CPS1 deficiency and more.
What is the significance of CRISPR gene editing in treating genetic diseases?
CRISPR gene editing has changed genetic medicine. It allows for precise genome changes, giving hope for treating diseases once thought incurable.
References
- “Infants with rare, incurable diseases are first to successfully receive personalized gene therapy treatment. NIH News Release. May 15, 2025. National Institutes of Health (NIH)
- “World’s First Patient Treated with Personalized CRISPR Gene Editing Therapy at Children’s Hospital of Philadelphia. Children’s Hospital of Philadelphia / Penn Medicine Newsroom. May 15, 2025. Children’s Hospital of Philadelphia
- “Gene-editing therapy made in just 6 months helps baby with life-threatening disease. Science news article. Science
- “Personalized CRISPR Therapy Successfully Treats Infant With Rare, Incurable CPS1 Deficiency. BioPharm International. May 16, 2025. BioPharm International
- “Baby With Rare Disease Gets Personalized Gene Therapy. NIH News in Health. July 2025. NIH News in Health
- “World’s first personalized CRISPR therapy given to baby. Nature Briefing / Nature News. Nature










