Last Updated on November 20, 2025 by Ugurkan Demir
A major breakthrough has been made in treating sickle cell disease. The CRISPR-Cas9 gene editing technology was used for the first time. The first sickle cell gene therapy patient has shown remarkable improvement, marking a turning point in genetic medicine.
The treatment changed the patient’s blood stem cells with CRISPR-Cas9 and then put them back in. This greatly reduced the symptoms of sickle cell anemia. This success is a big step forward in treating this serious disease.
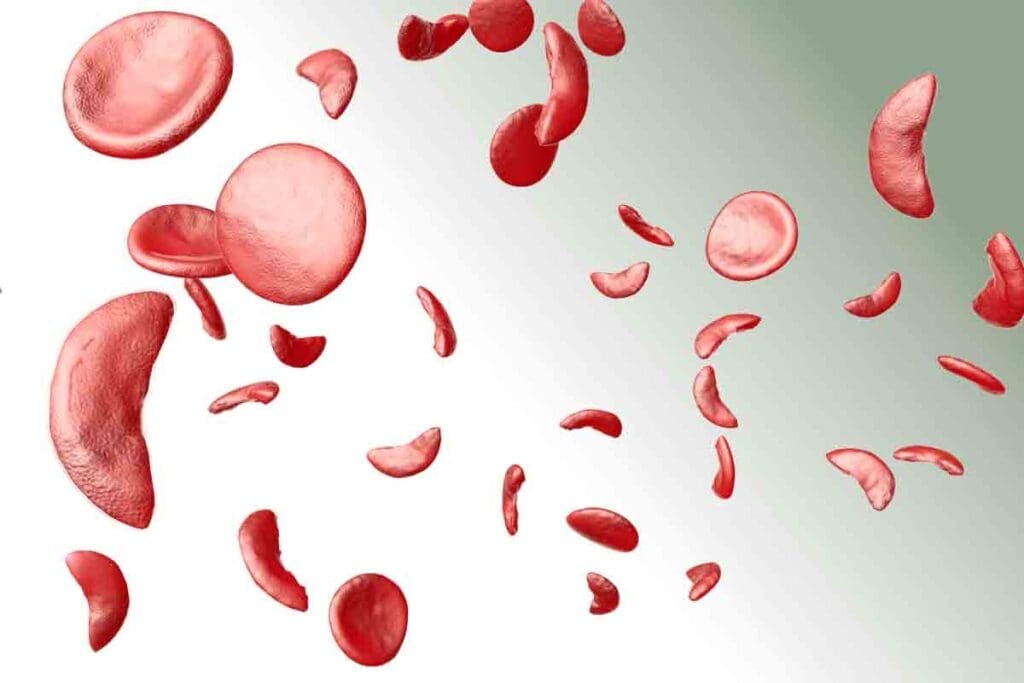
To understand sickle cell disease, we must explore its genetic roots and symptoms. It’s a group of inherited disorders that affect hemoglobin. This causes red blood cells to take on a sickle shape.
Sickle cell disease comes from a mutation in the HBB gene. This gene codes for a part of hemoglobin. The mutation leads to abnormal hemoglobin, known as sickle hemoglobin or HbS.
When someone gets two copies of this mutated gene, they often get the disease. The genetic defect changes a part of the beta-globin chain. This change causes hemoglobin to stick together under low oxygen, making red blood cells sickle-shaped.
People with sickle cell disease face many symptoms. These include:
Complications can be serious. They may include stroke, acute chest syndrome, and splenic sequestration. How often and how severe these complications are can vary a lot.
Traditional treatments aim to manage symptoms and prevent complications. Common methods include:
| Treatment | Description |
| Hydroxyurea | A medication that reduces the frequency of painful crises and may decrease the need for blood transfusions. |
| Blood Transfusions | Regular transfusions can help reduce the risk of complications by decreasing the number of red blood cells that can sickle. |
| Pain Management | Effective management of pain is critical, often involving a combination of medications and other interventions. |
Early diagnosis and complete care are key to improving life for those with sickle cell disease. While these traditional methods can manage the disease, they don’t cure it.
Gene therapy for sickle cell disease has seen big changes. New genetic editing tools have opened up new ways to treat this disease. This is good news for those who suffer from it.
At first, finding ways to fix sickle cell disease with gene therapy was tough. Scientists had to figure out how to edit genes correctly and get the treatment to work. These problems made it hard to make progress.
The arrival of CRISPR-Cas9 technology was a game-changer. It lets scientists edit the genes that cause sickle cell disease with great precision. This was a major step forward.
CRISPR-Cas9 is seen as a game-changer. It makes it possible to fix genetic problems like sickle cell disease by changing the genes themselves. Experts say it’s a big deal for treating genetic diseases.
Getting to clinical trials was a big step for gene therapy for sickle cell disease. After lots of testing in the lab, scientists started testing it on people. They wanted to see if it was safe and worked well.
These trials have shown great promise. Patients have seen big improvements. This is good news for getting these treatments approved and available to more people.
CRISPR-Cas9 technology has changed the game in gene editing. It brings unmatched precision and speed. This tool is a game-changer for genetic research and treatments, like those for sickle cell disease.
CRISPR-Cas9 finds and edits DNA with amazing accuracy. The Cas9 enzyme cuts the DNA at the right spot. This lets scientists fix or replace the faulty gene.
This method is super precise. It can target specific genes without messing with others. This reduces the chance of unwanted side effects in gene therapy.
CRISPR-Cas9 is better than old gene editing methods. It can be programmed to find specific genes. This makes it great for many genetic projects.
Sickle cell disease comes from a mutation in the HBB gene. CRISPR-Cas9 can fix this mutation, possibly curing the disease. Clinical trials have shown promising results, with patients seeing big improvements.
Using CRISPR-Cas9 for sickle cell disease is a big step forward in gene therapy. It directly tackles the disease’s genetic cause. This gives new hope to those affected by it.
The first sickle cell gene therapy patient was a big step forward. This case showed new ways to treat sickle cell disease. It also gave insights into gene therapy’s power.
The patient had been dealing with sickle cell disease for a long time. They tried many treatments but didn’t see much improvement. This made everyday life very hard.
Medical History: The patient had many pain crises and was in the hospital often. This was because of sickle cell disease.
The patient talked a lot with doctors before joining the trial. They knew about the good and bad sides of gene therapy. They chose to try it because they had no other good options.
Motivations for Participation: The patient hoped for a cure or a big improvement. They also wanted to help others with the same disease.
Before the gene therapy, the patient had many tests. These tests checked their health and whether they could get the treatment.
| Test | Purpose | Outcome |
| Blood Tests | Assess overall health and detect any abnormalities | Identified specific genetic markers related to sickle cell disease |
| Genetic Screening | Confirm the genetic basis of sickle cell disease | Confirmed the presence of the sickle cell gene mutation |
| Cardiac Evaluation | Assess heart function to ensure suitability for treatment | Revealed no significant cardiac issues |
The tests were key to seeing if the patient could join the trial. They also got ready for the treatment.
Gene therapy for sickle cell disease involves several steps. It starts with harvesting blood stem cells. This is key because it gives the cells needed to fix the disease’s genetic issue.
The first step is mobilization therapy. It gets the bone marrow to release stem cells into the blood. Then, these cells are collected through apheresis.
This method draws blood, separates the stem cells, and returns the rest to the patient.
The collected stem cells go to a lab for gene editing. They use CRISPR-Cas9 technology to fix the HBB gene. This aims to fix the mutation causing sickle cell disease and restore normal hemoglobin production.
Before the edited cells are put back, the patient gets conditioning chemotherapy. This clears the bone marrow for the new cells. After, the edited stem cells are reinfused into the blood.
They then go to the bone marrow to start making healthy red blood cells. The whole process is designed to be safe and effective, with close monitoring at every step.
Gene therapy for sickle cell disease has shown great promise in clinical trials. Patients have seen big improvements in their quality of life. This treatment aims to fix the disease at its source, which could be a cure.
Right after treatment, patients noticed fewer painful episodes. This is a key symptom of sickle cell disease. They also saw their blood counts return to normal, showing healthier blood production. These quick results show how well the gene editing works.
Follow-up data over time has backed up the initial good news. Patients have had fewer hospital stays for sickle cell crises. They also kept getting better overall. These long-lasting results show gene therapy’s lasting benefits.
A patient shared how gene therapy changed their life: “I felt less pain and had more energy after the treatment. I can now do things I couldn’t before because of my condition.”
This story shows the real-life benefits of gene therapy for sickle cell disease. It highlights the human side of these medical breakthroughs.
The FDA approved Casgevy and Lyfgenia in 2023. This was a big win for sickle cell disease patients. It brought hope to those suffering from this serious condition.
The FDA went through a lot to approve Casgevy and Lyfgenia. They did many clinical trials to check if these gene therapies were safe and worked well. These trials had different stages, from early studies to big Phase III trials.
Key Stages in the Approval Process:
Casgevy uses CRISPR-Cas9 technology to fix sickle cell disease in patients. It edits the patient’s stem cells to make healthy hemoglobin.
Benefits of Casgevy:
Lyfgenia uses a lentiviral vector to give the HBB gene to the patient’s stem cells. This helps make normal hemoglobin, easing sickle cell disease symptoms.
Advantages of Lyfgenia:
The FDA’s approval of Casgevy and Lyfgenia is a big step forward. These gene therapies offer new hope for sickle cell disease patients. They could greatly improve patient outcomes and reduce the disease’s impact.
| Therapy | Mechanism | Benefits |
| Casgevy | CRISPR-Cas9 gene editing | Potential cure, reduction in vaso-occlusive crises, improved quality of life |
| Lyfgenia | Lentiviral vector gene delivery | Effective in reducing symptom severity, long-term efficacy, well-tolerated safety profile |
In 2024, sickle cell disease treatment is seeing big steps forward. Ongoing clinical trials are working on making gene therapy better and safer. This gives hope to those dealing with this tough condition.
Many trials are looking into gene therapy for sickle cell disease. They’re checking how safe and effective it is.
The early results from these trials are looking good. Many patients are seeing big improvements. Gene therapy might even offer a functional cure for some, helping them live without the constant pain of sickle cell crises.
Here’s a quick look at the early findings:
| Trial Name | Therapy Type | Patient Outcomes |
| Trial XYZ | CRISPR-Cas9 | 90% reduction in vaso-occlusive crises |
| Trial ABC | Casgevy | 85% of patients achieved freedom from severe pain crises |
Patients in these trials are seeing big improvements. Many are enjoying a better quality of life. They’re no longer needing blood transfusions and pain meds as much. This change is also boosting their mental health, giving them hope for a cure.
As research keeps moving forward, doctors are hopeful about gene therapy for sickle cell disease. The trials and early results are key steps toward making these treatments available to more people.
Gene therapy is a new hope for sickle cell disease patients. It uses CRISPR-Cas9 technology, showing great promise in trials. But it raises many ethical questions.
The ethics of gene editing include respect for patients, not harming them, doing good, and fairness. It’s key that patients can make their own choices about gene therapy.
Gene therapy is expensive, making it hard for many to access. This raises big questions about fairness in healthcare.
| Therapy | Cost | Accessibility |
| Casgevy | $1.8 million | Limited |
| Lyfgenia | $2.0 million | Limited |
It’s important to make sure everyone can get gene therapy, no matter their money or where they live. Ways to help include lowering costs, better insurance, and teaching more about gene therapy.
By tackling these issues, we can make sure gene therapy is available to all sickle cell disease patients. Not just those who can pay for it.
Pioneering medical institutions are leading the way in gene therapy for sickle cell disease. They are creating new treatments and working with global experts to improve patient care.
Liv Hospital is a key player in gene therapy for sickle cell disease. They use cutting-edge technology and focus on the patient. This approach brings hope to those with this disease.
Their program includes a detailed pre-treatment check, precise gene editing, and careful post-treatment care. This ensures the best results for patients.
Many global centers of excellence are teaming up with Liv Hospital to improve gene therapy. They are doing groundbreaking research and trials to understand gene editing better.
| Institution | Focus Area | Notable Achievements |
| Liv Hospital | Gene Therapy | Pioneering gene editing techniques |
| Center for Gene Therapy | Clinical Trials | Successful trial outcomes |
| Global Research Institute | Basic Research | Advancements in gene editing technology |
Collaborative research is key in fighting sickle cell disease. By working together, medical institutions can speed up the development of treatments. These efforts often involve international partnerships, ensuring research is quickly applied in clinics.
One example is the global registry for gene therapy outcomes. It aims to track the long-term success and safety of these treatments. This registry will be a valuable resource for researchers and doctors, helping to improve treatment plans.
Gene therapy has changed how we treat sickle cell disease. It brings new hope to patients. This technology uses advanced tools like CRISPR-Cas9 to fix the disease’s genetic cause.
In 2023, the approval of Casgevy and Lyfgenia was a big step forward. These treatments are more effective and focused. With ongoing trials showing good results, the future looks bright for sickle cell disease management.
Places like Liv Hospital are leading in gene therapy research and treatment. Their work is key to making these new treatments available to those who need them.
Gene therapy is set to change sickle cell disease treatment for the better. It promises to improve patients’ lives and outcomes. The medical world is excited to see how it will evolve.
Sickle cell disease is a genetic disorder. It’s caused by a mutation in the HBB gene. This mutation leads to abnormal hemoglobin and sickle-shaped red blood cells.
Gene therapy for sickle cell disease edits blood stem cells. It uses CRISPR-Cas9 technology to fix the genetic mutation. Then, the edited cells are reinfused into the patient.
CRISPR-Cas9 is a tool for precise gene editing. It’s used in sickle cell disease to correct the HBB gene mutation. This effectively cures the disease.
The first patient’s identity is not shared publicly. Their case was a major milestone. It showed gene therapy’s promise in curing sickle cell disease.
Casgevy and Lyfgenia are gene therapies for sickle cell disease. They went through strict clinical trials. These trials showed they are safe and effective, earning FDA approval.
Gene therapy could cure sickle cell disease. This means no more managing symptoms. It also reduces the risk of complications.
Yes, many trials are ongoing. They’re exploring new gene therapies and improving current ones. Early results look promising.
Ethical issues include making sure everyone can access gene therapy. There are also concerns about cost and the ethics of gene editing.
Liv Hospital is a leader in sickle cell gene therapy. They’re doing research, working with others, and finding new treatments.
The future looks bright. Gene therapy is changing how we manage sickle cell disease. It could lead to a cure and better lives for patients.
CRISPR-Cas9 has been shown to be safe in sickle cell disease trials. Ongoing monitoring ensures it remains safe and effective over time.
Gene therapy could greatly improve sickle cell patients’ lives. It could reduce or eliminate symptoms. This would allow for a more normal life.
Subscribe to our e-newsletter to stay informed about the latest innovations in the world of health and exclusive offers!