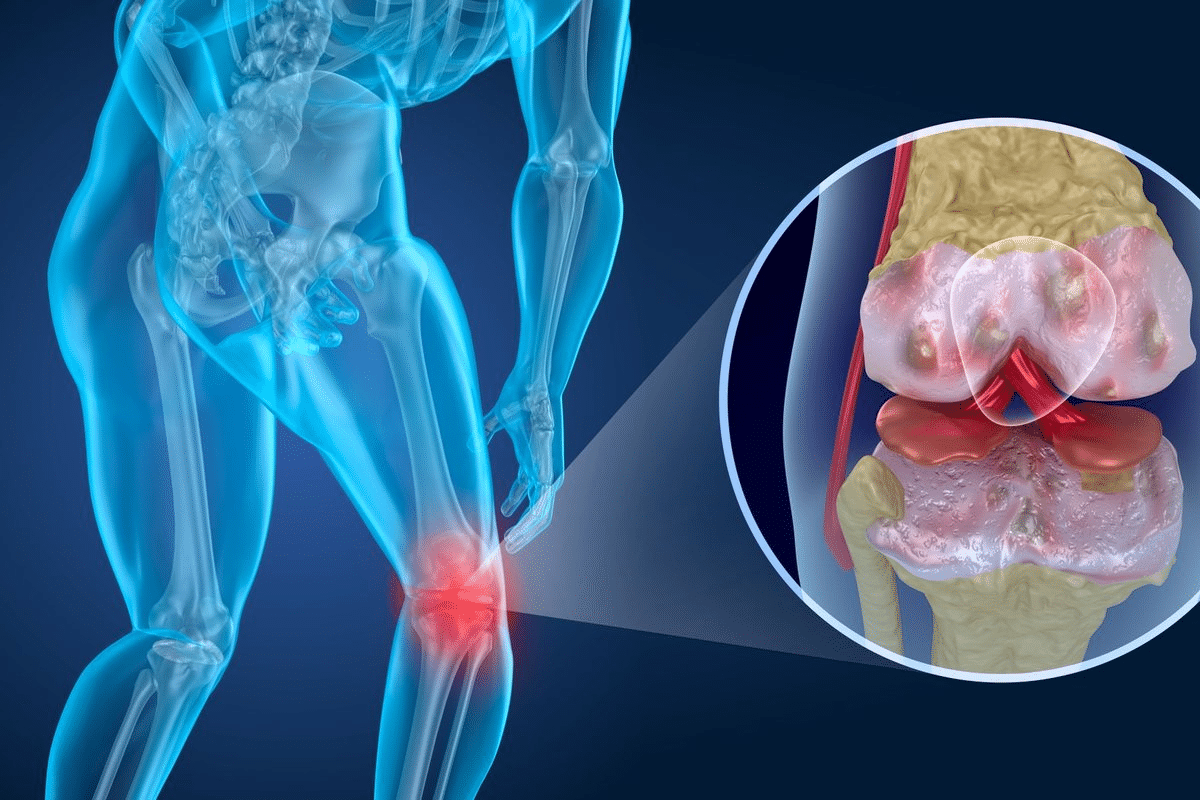
Rheumatoid arthritis (RA) is a chronic autoimmune disease. It affects about 0.2-1% of the world’s population. This condition causes a lot of disability and lost work due to ongoing inflammation in the joints. Explaining the five key immune mechanisms involved in the patho of rheumatoid arthritis and joint destruction.
Understanding the complex pathogenesis of RA is key for early detection and prevention. Recent studies have shown that RA is caused by a mix of genetic, epigenetic, and environmental factors.
At top medical centers like Liv Hospital, researchers are working hard to understand RA. They are looking into the five main ways this disease works. This could lead to new ways to treat it.
Rheumatoid arthritis (RA) is a big problem worldwide. It affects many people and puts a lot of pressure on healthcare systems. RA is not just a joint disease; it’s a complex condition that impacts patients and healthcare providers greatly.
RA is common, affecting up to 1% of the global population. By mid-century, the number of RA cases could reach over 31 million. This highlights the urgent need for more research into RA’s causes and effects.
RA often starts with pain and swelling in many joints at the same time. If not treated, it can cause serious damage and make it hard to move. Other symptoms, like nodules and vasculitis, can also appear, making the disease even harder to manage.
|
Region |
Estimated Prevalence (per 100,000) |
Projected Cases by 2050 |
|---|---|---|
|
North America |
900-1,000 |
4 million |
|
Europe |
800-900 |
3.5 million |
|
Asia-Pacific |
500-700 |
6 million |
It’s important to understand RA’s spread, symptoms, and how it progresses. By studying RA, we can find better ways to manage it. This will help us tackle the challenges RA poses to those affected and healthcare systems.

It’s key to grasp the basics of rheumatoid arthritis to find good treatments. RA is a complex disease where the body attacks its own joints. This leads to inflammation, cartilage loss, and bone damage.
In RA, the body makes antibodies against itself. About 67% of people with RA have these autoantibodies. They help doctors diagnose RA and show the disease is likely to get worse.
The main issue in RA is the inflammation in the joints. This causes the joint lining to grow too much. This growth damages the cartilage and bones.
The joints are where RA inflammation happens. Immune cells get into the joint lining. They make substances that keep the inflammation going.
RA’s cause involves genetics, environment, and the immune system. The table below shows what contributes to the disease:
|
Factor |
Description |
Impact on RA |
|---|---|---|
|
Genetic Factors |
HLA-DRB1 and other genetic risk factors |
Increased susceptibility to RA |
|
Environmental Triggers |
Smoking, infections, and other environmental exposures |
Triggers autoimmune response |
|
Immune Cells |
T cells, B cells, and other immune cells |
Perpetuate inflammation and joint damage |
Knowing these factors helps in making treatments that can help manage RA. This improves life for those with the disease.
Rheumatoid arthritis (RA) develops from a mix of genetic and environmental factors. This complex mix is key to understanding RA’s pathogenesis.
Genetics are a big part of RA’s pathogenesis. The HLA-DRB1 gene is a major focus. Some HLA-DRB1 alleles increase RA risk. Genes like PTPN22 and STAT4 also play a role.
These genes help control the immune system. But, they can lead to autoimmune issues seen in RA. Having certain genes can make you more likely to get RA.
Genetics aren’t the only factor. Environmental factors also play a big role. Smoking is a known environmental risk for RA, more so for those with certain genes. Infections and diet can also be triggers.
Exposure to these factors can change how genes work. These changes, called epigenetic modifications, can affect how genes are turned on or off. For example, smoking can make genes that cause inflammation work harder.
The mix of genetics and environment in RA is complex. Knowing this helps us find better treatments. Important points include:
By understanding the genetic and environmental mix, we can improve how we manage RA.
In RA, autoantibodies like ACPAs are key. They show a breakdown in immune tolerance and make the disease worse. These antibodies attack the body’s own proteins, causing inflammation and damage.
ACPAs target citrullinated proteins in RA. They are important for diagnosis and show a more severe disease. Having ACPAs means a higher risk of serious joint damage and a worse outcome.
Research shows ACPAs can appear years before symptoms start. This means they are vital in the early stages of RA.
Rheumatoid factor (RF) is common in RA patients. It’s not as specific as ACPAs but helps in diagnosis. Other autoantibodies, like anti-carbamylated protein antibodies, are also found in RA.
|
Autoantibody |
Target |
Significance |
|---|---|---|
|
ACPA |
Citrullinated proteins |
Highly specific for RA, associated with disease severity |
|
RF |
IgG Fc region |
Common in RA, used in diagnosis |
|
Anti-carbamylated protein |
Carbamylated proteins |
Associated with disease activity and severity |
ACPAs and other autoantibodies in RA show a loss of immune tolerance. This loss lets the immune system attack itself, causing ongoing inflammation and joint damage.
Knowing how autoantibodies are made and what ACPAs mean is key. It helps in finding better ways to diagnose and treat RA.
Immune cell dysfunction is a key feature of rheumatoid arthritis. It leads to ongoing inflammation and damage to joints. In RA, the immune system doesn’t work right, causing cells to act abnormally.
T cells are vital in RA’s development. They spot antigens in the joint tissue, get activated, and start a chain of events. This chain leads to the release of cytokines, which cause inflammation.
The way T cells change into different types is also important. In RA, there’s too much of the bad types and not enough of the good ones. This imbalance makes the immune response worse.
B cells are also key in RA. They turn into plasma cells and make autoantibodies like rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs).
These autoantibodies are not just markers of the disease. They also help start inflammation in the joints. This happens when they form complexes that deposit in the joints.
Innate immune cells, like macrophages and dendritic cells, also have big roles in RA. They make cytokines and chemokines that keep inflammation going. They also help turn on the adaptive immune cells.
|
Immune Cell Type |
Role in RA Pathogenesis |
Key Cytokines/Products |
|---|---|---|
|
T Cells |
Activation and differentiation driving inflammation |
IL-17, IFN-γ |
|
B Cells |
Autoantibody production |
RF, ACPAs |
|
Macrophages |
Cytokine production and inflammation |
TNF-α, IL-1β |
In conclusion, the way immune cells work is very important in rheumatoid arthritis. Knowing how different cells work together can help find new ways to treat the disease.
Understanding inflammatory cytokine networks is key to understanding RA. These cytokines drive the disease, causing joint inflammation and damage.
We’ll look at the main cytokines in RA, like TNF-alpha, IL-1, IL-6, and IL-17. We’ll explore their roles and how they interact.
TNF-alpha and IL-1 are pro-inflammatory cytokines found in high levels in RA patients. They start inflammation by making more cytokines, chemokines, and adhesion molecules. This brings immune cells to the synovial tissue.
TNF-alpha is a key player in RA’s inflammatory response. It triggers the production of IL-1, IL-6, and other cytokines. This creates a chain of inflammatory events. Blocking TNF-alpha is a successful treatment for RA.
|
Cytokine |
Function |
Role in RA |
|---|---|---|
|
TNF-alpha |
Pro-inflammatory |
Promotes joint inflammation and damage |
|
IL-1 |
Pro-inflammatory |
Contributes to synovial inflammation and bone erosion |
IL-6 is vital in RA, involved in both acute and chronic inflammation. It helps activate and differentiate T cells and B cells, fueling the autoimmune response.
The IL-6 pathway is complex, with both membrane-bound and soluble IL-6R signaling. This complexity offers many targets for treatment.
IL-17, made by Th17 cells, is important in RA. It boosts inflammation and joint damage. It works with TNF-alpha and IL-1 to increase inflammation.
Other inflammatory mediators, like chemokines and prostaglandins, also play a part in RA. Knowing how these interact is key to finding effective treatments.
By focusing on inflammatory cytokine networks, we can create new ways to manage RA better.
Mucosal dysbiosis and barrier dysfunction are key in rheumatoid arthritis (RA) onset. The gut and oral cavity mucosal surfaces are vital in autoimmune disease development, including RA.
The gut microbiota keeps the immune system in balance. In RA, the gut microbiota changes, or dysbiosis, occur. This can increase citrullinated proteins, starting autoimmunity.
Dysbiosis and Autoimmunity: An imbalance in gut microbiota leads to autoantibodies, like anti-citrullinated protein antibodies (ACPAs), seen in RA. RA patients have different gut microbiota than healthy people.
Periodontal disease, with Porphyromonas gingivalis infection, raises RA risk. P. gingivalis can citrullinate proteins, possibly triggering ACPAs.
The Role of P. gingivalis: Studies show P. gingivalis infection can cause ACPAs, fueling RA’s autoimmune response. This shows periodontal health’s role in RA.
Mucosal barrier dysfunction lets microbial products and inflammatory mediators into the blood. This causes systemic inflammation.
Consequences of Barrier Dysfunction: Barrier breakdown spreads inflammatory signals, worsening the autoimmune response and RA development. This understanding offers new RA treatment targets.
Research is uncovering how mucosal health affects RA. Studying mucosal dysbiosis and barrier dysfunction could lead to new RA treatments.
Understanding rheumatoid arthritis (RA) is key to finding better treatments. By knowing how RA works, we can create new ways to fight it. These methods aim to calm the immune system and lessen inflammation.
For years, disease-modifying antirheumatic drugs (DMARDs) have been a mainstay in RA treatment. Methotrexate is a top choice, known for its power to reduce inflammation and slow disease growth. Other DMARDs like sulfasalazine, hydroxychloroquine, and leflunomide also play important roles.
These drugs work in different ways. Some block folate metabolism, while others tweak the immune system or cut down on inflammatory chemicals.
|
Conventional DMARD |
Mechanism of Action |
Common Side Effects |
|---|---|---|
|
Methotrexate |
Inhibits folate metabolism, suppresses inflammation |
Nausea, fatigue, liver toxicity |
|
Sulfasalazine |
Modulates immune response, reduces inflammation |
Gastrointestinal upset, headache |
|
Hydroxychloroquine |
Interferes with antigen processing and presentation |
Retinal toxicity, gastrointestinal upset |
Biologic therapies have changed the game for RA treatment. TNF-alpha inhibitors like etanercept and adalimumab are big winners in fighting inflammation and slowing disease. Other biologics target different areas, like IL-6 (tocilizumab), IL-1 (anakinra), and B cells (rituximab).
New treatments for RA include JAK inhibitors like tofacitinib and baricitinib. They block inflammation by targeting Janus kinase pathways. Therapies aimed at IL-17 and IL-23, such as secukinumab and ustekinumab, are also on the horizon.
As we learn more about RA, we’ll see even better treatments. These new options offer hope for those who haven’t found relief yet.
Understanding rheumatoid arthritis (RA) is key to better treatment and care. We’ve looked at five main ways RA starts, including genetics, environment, and inflammation. These factors all play a role in how RA develops.
Using this knowledge in healthcare helps us give patients more tailored care. This means treatments can be made to fit each person’s needs. This approach can lead to better disease management.
As we learn more about RA, we can make treatments more precise. This will help improve the lives of people with RA all over the world. Our goal is to provide top-notch healthcare, and knowing how RA starts is a big part of that.
Rheumatoid arthritis is a chronic disease that causes joint inflammation and damage. It affects millions worldwide, putting a big strain on healthcare systems.
Rheumatoid arthritis is caused by a mix of genetics, epigenetics, and environment. It involves autoantibodies, immune cell dysregulation, and inflammation. Microbiome imbalance also plays a role.
Genes like HLA-DRB1 and environmental factors like smoking trigger rheumatoid arthritis. They lead to immune system changes and autoantibody production.
Autoantibodies, like anti-citrullinated protein antibodies (ACPAs), are key in diagnosing and progressing rheumatoid arthritis. They show a break in immune tolerance and contribute to the disease’s autoimmune nature.
Immune cells, such as T cells and B cells, cause chronic inflammation and joint damage. They activate, differentiate, and produce pro-inflammatory cytokines.
Treatments include conventional DMARDs, biologic therapies, and emerging approaches. These aim to modulate the immune system and reduce inflammation.
Knowing how rheumatoid arthritis works is key to better patient care. It guides the development of targeted therapies and improves disease management.
Mucosal origins and microbiome dysbiosis, like gut microbiota changes and periodontal disease, contribute to systemic inflammation and rheumatoid arthritis.
Inflammatory cytokine networks, including TNF-alpha and IL-6, keep inflammation going in rheumatoid arthritis. This leads to joint damage.
National Center for Biotechnology Information. Rheumatoid Arthritis: Understanding Disease Progression. Retrieved from https://pubmed.ncbi.nlm.nih.gov/26545940/
Subscribe to our e-newsletter to stay informed about the latest innovations in the world of health and exclusive offers!