Last Updated on December 1, 2025 by Bilal Hasdemir
Understanding the Inner Cell Mass of Blastocyst and Other Sources
Stem cells are key in regenerative medicine, with the power to change how we treat diseases. A big source of these cells is the inner cell mass of blastocyst. This is an early embryo stage with pluripotent cells that can turn into any cell in our body.
The journey of embryonic stem cells from the inner cell mass is complex. It involves carefully taking and growing these cells. Knowing where stem cells come from, like from embryos and others, is vital for medical progress.
Key Takeaways
- The inner cell mass of blastocyst is a primary source of embryonic stem cells.
- Embryonic stem cells are pluripotent, capable of developing into any cell type.
- Understanding the sources of stem cells is essential for regenerative medicine.
- Different sources of stem cells include embryonic stem cells and other types.
- The extraction and culturing of embryonic stem cells is a complex process.
The Fundamental Nature of Stem Cells
Stem cells are at the core of regenerative medicine. They can turn into many different cell types. This makes them key for studying development, fixing tissues, and understanding diseases.
Defining Stem Cells and Their Unique Properties
Stem cells can grow more of themselves and turn into different cell types. Self-renewal means they can keep their numbers by dividing. Differentiation is when they become specific cells, like nerve or muscle cells. This is why they’re so important for growth and keeping tissues healthy.
Stem cells have special traits:
- Pluripotency: They can become any type of body cell.
- Self-renewal: They can keep their numbers by dividing.
- Differentiation: They can turn into specific cell types.
The Importance of Stem Cells in Medicine and Research
Stem cells are a big hope for medical science and treating diseases. They can become many cell types. This makes them very useful for fixing damaged tissues, engineering new tissues, and cell therapy.
| Application | Description | Potential Benefits |
| Regenerative Medicine | Repairing or replacing damaged tissues and organs. | Treatment of degenerative diseases, such as Parkinson’s disease and diabetes. |
| Tissue Engineering | Creating functional tissue substitutes. | Repair of damaged heart tissue, skin grafts, and organ transplantation. |
| Cell Therapy | Using stem cells to replace or support damaged cells. | Treatment of blood disorders, immune system diseases, and certain cancers. |
The possibilities of stem cells in medicine and research are endless. They offer new ways to treat many conditions and help us understand human biology better.
Embryonic Stem Cells: The Original Source
Embryonic stem cells come from the early embryo. They can turn into many different cell types. This makes them very useful in medical research and could help in treatments.
Early Embryonic Development
Early embryonic development is complex. It starts with fertilization and ends with the formation of a blastocyst. Embryogenesis is the first stage, where the fertilized egg divides several times to form a blastocyst.
This stage is key. It’s where embryonic stem cells start to develop. Knowing about this stage helps researchers use these cells to their full advantage.
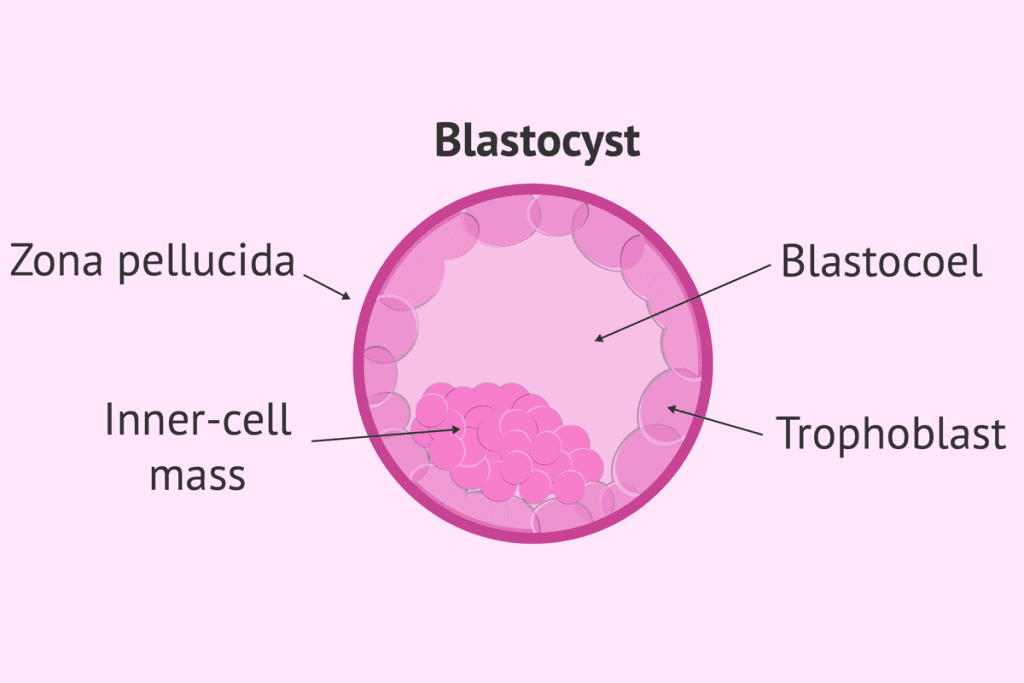
Formation of the Blastocyst
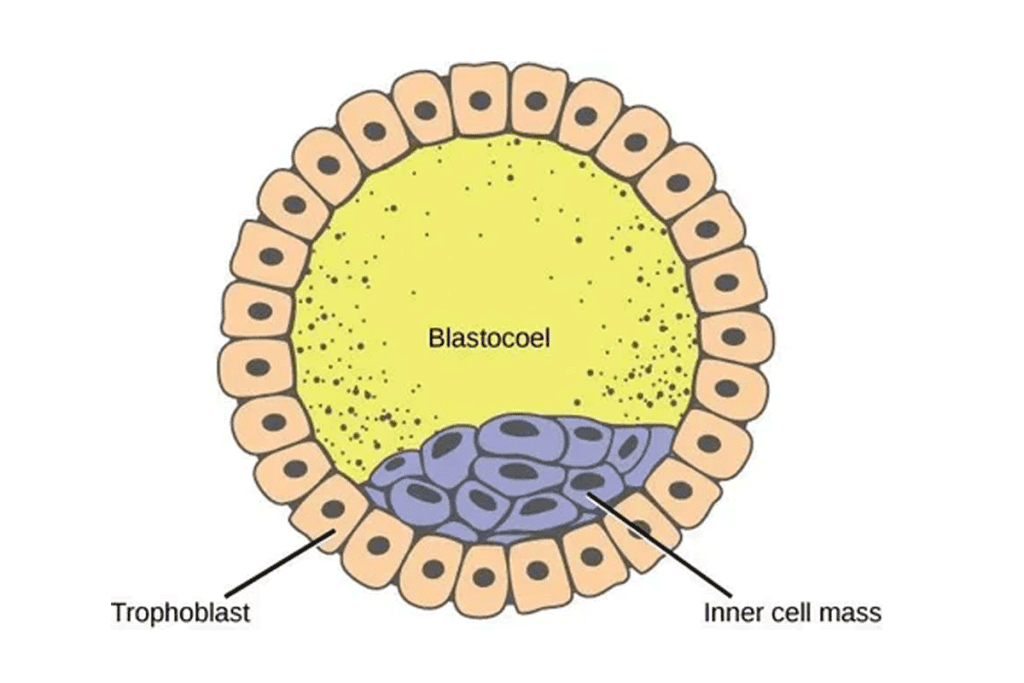
The blastocyst forms about 5-6 days after fertilization. It has two main parts: the trophectoderm and the inner cell mass (ICM). The ICM is important because it leads to the creation of embryonic stem cells.
The blastocyst’s formation is a big deal. It happens before the embryo implants in the uterus. This process involves cell divisions and differentiation, leading to the development of the embryoblast and trophoblast.
Ethical Considerations in Embryonic Stem Cell Research
Using embryonic stem cells in research brings up ethical questions. These cells usually come from embryos created during in vitro fertilization (IVF) that are not used for pregnancy.
There’s a big debate about the ethics of using embryos for research. Some think the benefits of this research are worth it, while others believe we should find other ways to avoid using embryos.
Researchers and those making policies are trying to find a balance. They want to move science forward while also respecting ethical limits.
The Inner Cell Mass of Blastocyst: Primary Source of Pluripotent Stem Cells
The inner cell mass of blastocyst is known for its ability to turn into different cell types. This makes it very important in stem cell research and regenerative medicine.
Structure and Formation of the Inner Cell Mass
The inner cell mass (ICM) forms early in embryonic development, around 4-5 days after fertilization. It’s a group of cells in the blastocyst that will become the fetus’s main structures.
Creating the ICM is a complex process. It involves many cell and molecular interactions. Special transcription factors help keep these cells pluripotent.
Pluripotency Characteristics of Inner Cell Mass Cells
ICM cells are pluripotent, meaning they can become any cell type. This is thanks to key transcription factors like Oct4, Sox2, and Nanog.
- They can self-renew and stay pluripotent.
- They can turn into many different cell types.
- They have specific markers that show their pluripotency.
Extraction and Cultivation Techniques
Getting stem cells from the ICM is a delicate process. It’s done to keep the cells pluripotent and undamaged. The methods include:
- Immuno-surgical isolation of the ICM.
- Culturing the ICM cells in special media that support their pluripotency.
- Keeping the cells in conditions that stop them from differentiating.
These methods are key for getting stable stem cell lines. These lines are used in research and could be used in treatments.
In Vitro Fertilization and Stem Cell Derivation
IVF is a common fertility treatment that creates embryos. Sometimes, these embryos are extra and can be used for stem cell lines. This happens because IVF mixes an egg with sperm outside the body, often making more embryos than needed.
The IVF Process
The IVF journey starts with ovarian stimulation. This uses medicine to make the ovaries produce many eggs. Then, these eggs are taken out and mixed with sperm in a lab.
The embryos grow for 3-5 days before being put back in the uterus.
Key Steps in IVF:
- Ovarian stimulation
- Egg retrieval
- Fertilization
- Embryo culture
- Embryo transfer
From Surplus Embryos to Stem Cell Lines
Extra embryos from IVF can be used for research. This gives us embryonic stem cells. These cells can turn into any cell type, which is very useful for research and treatments.
To get stem cell lines, we thaw the embryos, grow them, and take out the inner cell mass. Then, we grow the stem cells for research or treatments.
| Step | Description |
| Embryo Thawing | Surplus embryos are thawed and checked if they are alive. |
| Culturing | Embryos grow to let the inner cell mass expand. |
| Inner Cell Mass Isolation | The inner cell mass is taken out and grown to get embryonic stem cells. |
Getting stem cell lines from IVF embryos is a big step for research. It helps us understand human development and diseases. It also could lead to new treatments.
Adult Stem Cells: Reservoirs Throughout the Body
The human body has many adult stem cell reservoirs. These cells help keep tissues healthy by replacing damaged or dying cells. They are found in different tissues and organs.
Multipotent vs. Pluripotent Stem Cells
Adult stem cells are multipotent. This means they can turn into several cell types. But, they can’t become as many types as pluripotent stem cells can. For example, blood cells in the bone marrow come from these stem cells.
Knowing the difference between multipotency and pluripotency helps us understand adult stem cells. They are not as flexible as embryonic stem cells. Yet, they play a big role in fixing damaged tissues.
Tissue-Specific Adult Stem Cell Niches
Adult stem cells live in special areas called niches. These niches are in tissues and keep the stem cells ready to act. For example:
- The bone marrow, which houses hematopoietic stem cells.
- The basal layer of the epidermis, containing skin stem cells.
- The intestinal crypts, where intestinal stem cells reside.
These niches give stem cells the right environment to stay stem cells. They also help them respond to tissue needs.
Limitations and Advantages of Adult Stem Cells
Adult stem cells have big advantages. They are less debated than embryonic stem cells and have a lower risk of tumors. But, they are harder to grow in the lab. They also can’t turn into as many cell types.
| Characteristics | Adult Stem Cells | Embryonic Stem Cells |
| Differentiation Capability | Multipotent | Pluripotent |
| Source | Tissue-specific niches | Early embryos |
| Ethical Concerns | Less controversial | More controversial |
It’s key to know these traits for using adult stem cells in treatments and studies.
Bone Marrow: A Rich Source of Hematopoietic Stem Cells
Bone marrow is a key source of hematopoietic stem cells. These cells are vital for making blood cells. They help create red blood cells, white blood cells, and platelets.
Types of Stem Cells in Bone Marrow
Bone marrow has two main stem cell types: hematopoietic and mesenchymal. Hematopoietic stem cells turn into all blood cells. Mesenchymal stem cells can become different cell types, like bone and cartilage cells.
- Hematopoietic stem cells are key for blood cell creation.
- Mesenchymal stem cells help form various tissues.
Bone Marrow Harvesting Procedures
Bone marrow harvesting, or aspiration, collects stem cells. It’s done under local or general anesthesia. The stem cells can be used for autologous stem cell transplantation or allogenic transplantation.
- The patient is prepared for the procedure, which may involve anesthesia.
- Bone marrow is aspirated from the hip bone or another suitable location.
- The harvested bone marrow is then processed to isolate the stem cells.
Using bone marrow for stem cells has changed hematology and oncology. It allows for bone marrow transplants for patients with leukemia and other blood disorders.
Peripheral Blood Stem Cells: Mobilized Progenitors
Mobilized peripheral blood stem cells are a new hope for stem cell treatments. They are collected from the blood, not the bone marrow. This method is less invasive and is growing in popularity.
Mobilization Techniques
To get these stem cells, we first need to mobilize them. This means we make them move from the bone marrow into the blood. We use a drug called granulocyte-colony stimulating factor (G-CSF) to do this.
Mobilization regimens can change based on the patient and the treatment plan. G-CSF is key in making this process work better.
- G-CSF administration
- Plerixafor (a CXCR4 antagonist) for enhanced mobilization
- Combination regimens for optimal results
Collection Through Apheresis
After mobilizing the stem cells, we collect them using apheresis. This method separates the stem cells from other blood parts.
Apheresis is a minimally invasive way to get a lot of stem cells. These cells can be used for many treatments, like transplants and regenerative medicine.
Apheresis has many benefits. It’s safer than bone marrow harvesting and can collect stem cells quickly.
Umbilical Cord Blood: Preserving Birth-Related Stem Cells
Umbilical cord blood was once seen as waste. Now, it’s a key part of medicine, thanks to stem cell therapy. Collecting and storing this blood is a big deal in today’s medicine. It opens up new ways to treat and study diseases.
Collection and Banking Procedures
Getting umbilical cord blood is simple. It happens right after a baby is born. First, the cord is clamped and cut. Then, blood from the cord and placenta is taken out. This blood goes to a bank for safekeeping.
Cord blood banking procedures include a few steps:
- Collection: Blood is taken from the umbilical cord after it’s cut.
- Processing: The blood is split to get the stem cells out.
- Testing: The blood is checked for diseases and safety.
- Cryopreservation: Stem cells are frozen to keep them alive.
- Storage: Frozen stem cells are kept in special places for a long time.
Advantages of Cord Blood Stem Cells
Cord blood stem cells have big advantages for medical use:
- They are immunologically naive, which lowers the risk of disease.
- These stem cells are easy to find and store for a long time.
- They have a lower risk of viral transmission than other stem cells.
- Cord blood banks have a wide range of stem cells. This helps find a match for patients from different backgrounds.
Public vs. Private Cord Blood Banking
Choosing between public or private cord blood banking depends on your family’s health and what you prefer. Public cord blood banking means donating for anyone’s use. It helps a global registry. Private cord blood banking keeps the blood for the family’s use only.
Public banking is giving back, helping others. Private banking gives the family a special source of stem cells. This might lower the chance of rejection in future treatments.
Placental and Amniotic Fluid Stem Cells
There are more stem cell sources than just embryonic and adult ones. The placenta and amniotic fluid are rich in stem cells with special traits. Scientists are excited about these cells for their role in healing and building new tissues.
Characteristics of Placental Stem Cells
Placental stem cells come from the placenta, a vital organ in pregnancy. These cells can help the body in many ways. They can calm the immune system and turn into different cell types.
Key characteristics of placental stem cells include:
- Immunosuppressive properties, making them suitable for allogenic transplantation
- Ability to differentiate into multiple cell lineages
- Easy to isolate and expand in culture
| Characteristic | Description |
| Immunomodulation | Ability to modulate the immune system, reducing inflammation |
| Multipotency | Capacity to differentiate into various cell types, such as mesenchymal cells |
| Ease of Isolation | Can be readily isolated from the placenta post-partum |
Amniotic Fluid as a Stem Cell Source
Amniotic fluid, which surrounds the fetus, is also a stem cell treasure trove. These cells, called amniotic fluid stem cells (AFSCs), can turn into many different cell types.
“Amniotic fluid stem cells have the ability to differentiate into multiple lineages, including cells of the cardiovascular, muscular, and nervous systems.”-Researchers say.
Using amniotic fluid stem cells has many benefits. They are easy to get and don’t raise the same ethical questions as embryonic stem cells.
Advantages of amniotic fluid stem cells:
- Non-invasive procurement method
- High proliferative capacity
- Potential for use in tissue engineering and regenerative medicine
Adipose Tissue: An Abundant Source of Mesenchymal Stem Cells
Adipose tissue, or fat tissue, is now seen as a rich source of mesenchymal stem cells. These cells are key in regenerative medicine because of their ability to help heal and grow new tissues. They have many uses in treating diseases.
Fat Tissue Extraction Procedures
To get mesenchymal stem cells from fat tissue, several steps are needed. First, fat is taken out through liposuction. Then, the fat is processed to find the stem cells. This involves breaking down the tissue and spinning it to separate the cells.
This method is seen as minimally invasive. It’s done under local anesthesia, which lowers the risk of problems. Also, because there’s so much fat in our bodies, we can get a lot of stem cells. This makes fat tissue a great source for treatments.
Applications of Adipose-Derived Stem Cells
Adipose-derived stem cells are used in many ways in regenerative medicine. They can turn into different cell types, like fat cells and bone cells. This makes them good for fixing damaged tissues and for cosmetic and plastic surgery.
- Tissue repair and regeneration
- Cosmetic and plastic surgery
- Orthopedic treatments
- Wound healing
Using adipose-derived stem cells has benefits. They help the body accept new cells, which lowers the chance of rejection. Also, it’s easy to grow these cells in the lab. This makes them a good choice for treatments tailored to each person.
Induced Pluripotent Stem Cells (iPSCs): Reprogramming Somatic Cells
Cellular reprogramming has led to the creation of induced pluripotent stem cells. This is a big step forward in regenerative medicine and our understanding of cells.
Methods for Creating iPSCs
To make iPSCs, several steps are needed. First, you pick somatic cells. Then, you add reprogramming factors. Lastly, you grow the cells in a way that keeps them pluripotent. Many methods have been created to make this process better and safer.
- Selection of somatic cells: Typically, skin fibroblasts or blood cells are used.
- Introduction of reprogramming factors: This is usually done using viral vectors, though non-integrating methods are also being developed.
- Cultivation under pluripotency-supporting conditions: Specific culture media and conditions are used to maintain the pluripotent state.
| Method | Description | Advantages | Limitations |
| Viral Vector Method | Uses viruses to deliver reprogramming factors into somatic cells. | High efficiency | Risk of insertional mutagenesis |
| Non-Integrating Method | Uses non-viral vectors or episomal vectors to deliver reprogramming factors. | Safer, avoids genomic integration | Lower efficiency compared to viral methods |
Advantages and Limitations Compared to Embryonic Sources
iPSCs have big advantages over embryonic stem cells. They avoid ethical issues and can be made from a patient’s own cells for treatments. But, they also have downsides like the chance of epigenetic memory and tumorigenesis.
The creation of induced pluripotent stem cells is a major step in stem cell science. It opens up new ways for research and treatment. As this field grows, iPSCs will likely become more important in medical research and regenerative medicine.
Neurogenic Stem Cell Niches: Brain and Spinal Cord Sources
Neurogenic stem cell niches in the brain and spinal cord are key for new research and treatments. These niches are special areas filled with neural stem cells. These cells are vital for growing, keeping, and fixing the nervous system.
Neurogenesis and Neural Stem Cells
Neurogenesis is the creation of new brain cells. It’s a complex process that involves neural stem cells. These cells can turn into different types of brain cells, helping the brain grow and change.
Neural stem cells can make more of themselves and become different types of brain cells. They need their environment, or niche, to work right. This environment gives them the signals they need to stay healthy and function.
Challenges in Neural Stem Cell Harvesting
Getting neural stem cells from these niches is hard. It takes special skills and careful methods to get and grow these cells.
One big problem is accessibility. The niches are deep in the brain, making it hard to reach them without harming nearby tissue.
- Technical difficulties in isolating neural stem cells
- Limited understanding of niche-specific signals
- Ethical considerations regarding the source of neural stem cells
Future Frontiers in Stem Cell Sourcing
New technologies are changing how we get stem cells. Big steps are being made in this field. These changes will change how we use stem cells.
Emerging Technologies for Stem Cell Derivation
Genetic engineering and biomaterials science are leading the way. CRISPR/Cas9 gene editing is being looked at to make stem cells better.
- CRISPR/Cas9 gene editing for precise modification of stem cells
- Use of induced pluripotent stem cells (iPSCs) for disease modeling
- Development of biomaterials that support stem cell growth and differentiation
These new methods are making stem cell creation more efficient. They also open up new uses in regenerative medicine.
Synthetic and Bioengineered Stem Cell Sources
Creating synthetic and bioengineered stem cells is a big step forward. These new methods aim to make stem cells for specific treatments.
Some key areas of research include:
- Creating synthetic stem cell niches that mimic the natural environment
- Engineering stem cells for better tissue repair
- Creating artificial stem cell lines through reprogramming
These advances could solve problems with traditional stem cells. Issues like ethics and availability might be less of a problem.
The future of stem cell therapy looks bright. These new technologies could help treat many diseases and injuries.
Conclusion
Understanding stem cells is key to improving medicine and finding new treatments. We have different types of stem cells to study. These include cells from the early stages of development, adult tissues, and those made from adult cells.
As we learn more about these cells, the future of medicine looks bright. New technologies and ways to get stem cells will help us even more. This will make the field of regenerative medicine even more powerful.
By studying these stem cells, we can create new treatments. This will help patients live better lives. The work on stem cells shows how important it is to keep researching. It leads to new discoveries and better treatments.
FAQ
What is the inner cell mass of blastocyst?
The inner cell mass of blastocyst is a group of cells. It’s an early embryo stage. These cells grow into the fetus’s main parts and are a key source of stem cells.
What are embryonic stem cells derived from?
Embryonic stem cells come from the inner cell mass of blastocyst. They are often taken from extra embryos made during IVF.
What is the difference between pluripotent and multipotent stem cells?
Pluripotent stem cells, like those from the inner cell mass, can turn into any cell type. Multipotent stem cells, found in adults, can only turn into specific cell types.
How are induced pluripotent stem cells (iPSCs) created?
iPSCs are made by changing adult cells into a pluripotent state. This is done by adding special genes. This way, they can become different cell types.
What are the advantages of using umbilical cord blood as a source of stem cells?
Umbilical cord blood is a rich source of hematopoietic stem cells, which can be easily collected at birth and cryopreserved for future use. This makes it a valuable resource for treating certain blood and immune system disorders, especially in a clinical setting.”
What is the role of bone marrow in stem cell biology?
Bone marrow is where most blood stem cells are found. These cells make all blood types. Bone marrow is used in treatments for blood diseases.
How are peripheral blood stem cells mobilized and collected?
Peripheral blood stem cells are moved out of the body with special drugs. Then, they are collected using a process called apheresis. This separates the stem cells from other blood parts.
What are the potentials of adipose-derived stem cells?
Stem cells from fat tissue are being studied for regrowing tissues and controlling the immune system. They might help in fixing damaged tissues.
What are the challenges associated with harvesting neural stem cells?
Getting neural stem cells is hard. It’s because neural tissue is hard to reach, and there are many types of neural cells. Also, it takes special ways to get and grow these cells.
What is the significance of the blastocyst stage in embryonic development?
The blastocyst stage is very important. It’s when the inner cell mass forms, which grows into the fetus. It’s also when stem cells are usually taken.
How do emerging technologies impact stem cell derivation?
New technologies like synthetic biology and bioengineering are changing how we get stem cells. They help make new stem cell sources and improve old ways.
References:
Hassani, S. N., McDonald, K. L., Smith, J. L., & Latham, K. E. (2018). Transition of inner cell mass to embryonic stem cells. Stem Cell Research & Therapy, 9(1), Article 86. https://doi.org/10.1186/s13287-018-0843-1 PMC
- Discusses derivation and maintenance of embryonic stem cells from the inner cell mass of blastocysts, and how culture conditions influence pluripotency. PMC
Vazin, T., & Freed, W. J. (2010). Human embryonic stem cells: derivation, culture, and differentiation: A review. Stem Cell Reviews and Reports, 6(1), 25-43. https://doi.org/10.1007/s12015-009-9088-2 PMC
- Provides details on how embryonic stem cells are derived (from ICM), cultured, and differentiated. Useful to support your section on ESC origin and lab handling. PMC
De la Torre, P., Miah, S., & Andrés, M. (2020). Current status and future prospects of perinatal stem cells. Frontiers in Bioengineering and Biotechnology, 8, Article 972. https://doi.org/10.3389/fbioe.2020.00972 PMC
- Reviews perinatal stem cells (e.g. umbilical cord, placenta, amniotic fluid) as alternative sources”highlighting less ethical concern, availability, and their biological features. PMC
Allouh, M. Z., Al-Salemi, N., & AlHashmi, A. (2025). Mesenchymal stromal/stem cells from perinatal sources. Stem Cell Research & Therapy, 16, Article 188. https://doi.org/10.1186/s13287-025-04254-0 BioMed Central
- Focuses on MSCs isolated from perinatal sources (placenta, umbilical cord, etc.), comparing them to adult MSCs. Good for illustrating alternative sources beyond ESC. BioMed Central
Zakrzewski, W., Dobrzyş„ski, M., Szymonowicz, M., & Rybak, Z. (2019). Stem cells: Past, present, and future. Stem Cell Research & Therapy, 10, Article 68. https://doi.org/10.1186/s13287-019-1165-5 BioMed Central