Last Updated on December 1, 2025 by Bilal Hasdemir

A recent clinical trial has shown promising results for mesenchymal stem cell therapy. It helps alleviate symptoms of recessive dystrophic epidermolysis bullosa (RDEB). This is a rare genetic disorder.
Despite the promise, MSCs have been surrounded by controversy. This is because of the risks and unproven treatments they involve. The benefits of MSCs are being studied for many medical uses. But, it’s important to know the risks of stem cell therapy.
This article will explore the controversy around MSCs. It will highlight the need to understand stem cell therapy side effects. We also need rigorous testing to ensure treatments are safe and effective.
Key Takeaways
- The therapeutic benefits of MSCs are being explored for various medical applications.
- MSCs have shown promise in treating genetic disorders like RDEB.
- Understanding stem cell therapy risks is key for safe and effective treatments.
- Rigorous testing is necessary to mitigate possible side effects.
- The controversy around MSCs comes from their benefits and risks.
The Science Behind Mesenchymal Stem Cells
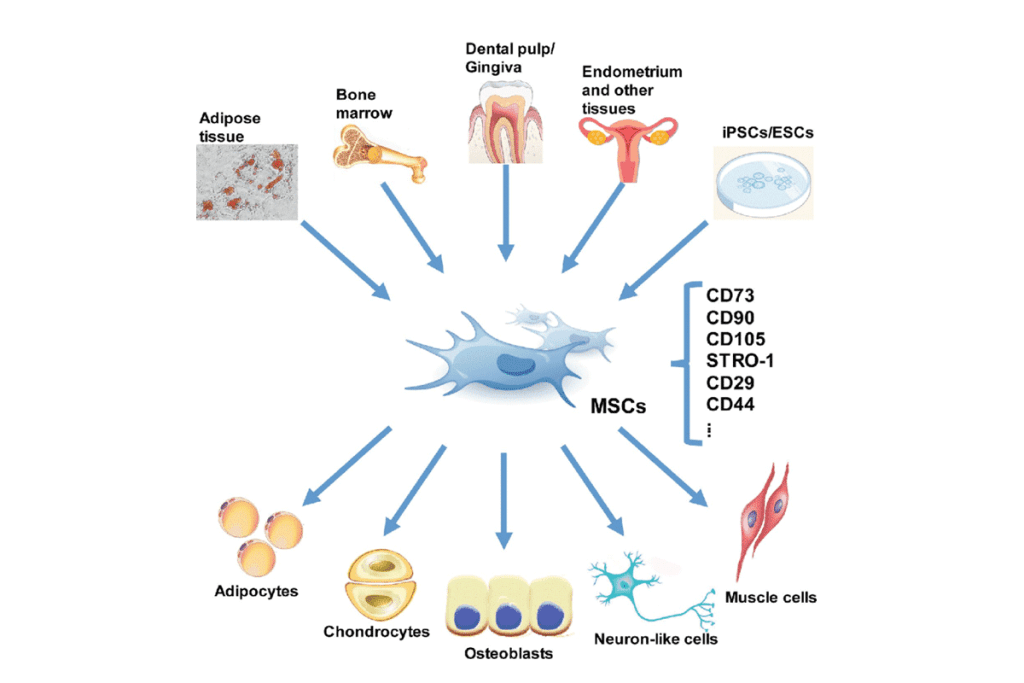
Mesenchymal stem cells (MSCs) are getting a lot of attention in medicine. They can turn into different cell types. This helps fix and grow tissues.
What Are Mesenchymal Stem Cells?
Mesenchymal stem cells can become osteoblasts, chondrocytes, and adipocytes. This makes them key in fixing and growing tissues.
They are special because they can:
- Differentiate into multiple cell types
- Modulate the immune system
- Support tissue regeneration through paracrine effects
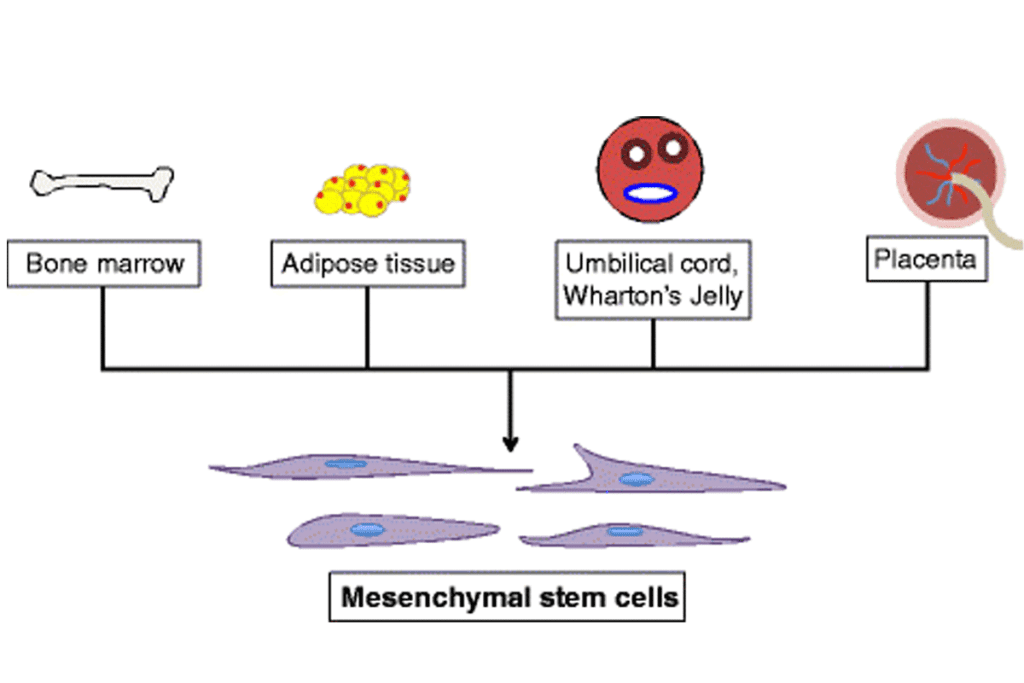
Sources and Types of MSCs
MSCs come from different places, like:
- Bone marrow
- Umbilical cord tissue
- Adipose tissue
Where MSCs come from affects their abilities. For example, those from umbilical cord tissue grow more and are more primitive.
Therapeutic Potentials and Mechanisms
MSCs can help fix tissues, reduce swelling, and help grow new ones. Studies show they work well for many diseases and injuries.
They help in several ways:
- Immunomodulation: They calm the immune system, helping healing.
- Tissue Regeneration: They can turn into cells that fix damaged tissues.
- Paracrine Effects: They release substances that help repair and grow tissues.
While early clinical trials suggest MSCs may be safe and effective, further long-term studies are needed.This opens doors for using them in medicine.
Historical Development of MSC Therapies
Understanding MSC therapies’ history is key to seeing their current uses. The path of MSCs from lab discoveries to treatments is long and complex.
Early Research and Discoveries
The idea of using MSCs for therapy started in the 1960s. Early studies showed they help form blood cells, setting the stage for their wider use.
In the 1980s and 1990s, research turned to MSCs’ role in fixing damaged tissues. This led to the first clinical trials, starting a new chapter in regenerative medicine.
Evolution of Clinical Applications
As research grew, MSCs’ uses expanded. They’re now studied for treating heart diseases, bone injuries, and autoimmune issues.
- Cardiovascular diseases: MSCs aim to fix heart damage.
- Orthopedic injuries: They help with bone and cartilage healing.
- Autoimmune disorders: MSCs’ ability to calm the immune system is explored for treating diseases like multiple sclerosis and lupus.
Shift from Research to Commercial Applications
MSC therapies moving from labs to the market has brought both benefits and challenges. Making them available to more people is a plus. But, it also raises worries about treatment quality and safety.
| Aspect | Research Setting | Commercial Application |
| Regulatory Oversight | Strict, following research rules and ethics | Varies, based on laws and rules |
| Treatment Quality | High, due to careful testing and environments | Can vary, based on how they’re made and checked |
| Accessibility | Limited, mainly for trials or special places | Wider, as companies make them more available |
The history of MSC therapies shows we need a careful balance. We must encourage new ideas while keeping patients safe and treatments effective.
Current Medical Applications of MSCs
Mesenchymal Stem Cells (MSCs) are a key part of regenerative medicine. They can turn into different cell types and help repair tissues. This makes them useful for treating many diseases and injuries.
FDA-Approved Treatments
There are a few FDA-approved MSC treatments out there. One example is for graft-versus-host disease (GvHD) after stem cell or bone marrow transplants. The FDA has okayed MSC therapies for this because they can calm down the immune system and help manage GvHD.
Experimental and Investigational Uses
MSCs are also being looked at for other conditions. These include:
- RDEB (Recessive Dystrophic Epidermolysis Bullosa), a rare genetic disorder that makes skin and mucous membranes fragile.
- Osteoarthritis, where MSCs might help grow new cartilage and improve joint function.
- Autoimmune disorders, where MSCs could help control the immune system.
- Cardiovascular conditions, where MSCs might repair damaged heart tissue.
These uses are in different stages of clinical trials. Some are showing very promising results.
Conditions Commonly Targeted
MSCs are being studied for many conditions because of their ability to repair tissues. Here’s a table that shows some of the main conditions being targeted with MSC therapies:
| Condition | Therapeutic Goal | Current Status |
| Graft-versus-Host Disease (GvHD) | Immunosuppression | FDA-Approved |
| RDEB | Tissue Repair | Experimental |
| Osteoarthritis | Cartilage Regeneration | Experimental |
| Autoimmune Disorders | Immune System Modulation | Experimental |
| Cardiovascular Diseases | Heart Tissue Repair | Experimental |
Using MSCs in these areas could change how we treat many diseases. As research goes on, we hope MSC therapies will become more common and effective for more conditions.
Stem Cell Therapy Side Effects: A Complete Overview
Stem cell therapy is growing, and knowing its side effects is key for safety. MSC therapy looks promising for many health issues. But, it’s important to know and understand the risks.
Common Adverse Reactions
MSC therapy can cause infusion-related reactions. These might include fever, chills, and fatigue. These reactions are usually mild and often go away with little treatment.
A study in the Journal of Translational Medicine found 30% of patients had these reactions. Keeping a close eye on patients during and after treatment can help lessen these issues.
| Adverse Reaction | Frequency | Management |
| Fever | Common | Antipyretics |
| Chills | Common | Supportive care |
| Fatigue | Very Common | Rest, hydration |
Rare but Serious Complications
MSC therapy can lead to tumor formation and immune reactions. These are rare but can be serious.
A review in Stem Cells Translational Medicine found cases of tumors in patients. This shows the need for long-term monitoring.
Long-Term Safety Concerns
Long-term safety of MSC therapy is a big concern. As it’s used more, studying long-term effects is vital.
Some research suggests MSCs might stay in the body for a long time. This could mean lasting benefits. But, more research is needed to understand this fully.
Understanding stem cell therapy side effects is complex. By looking at common issues, rare but serious problems, and long-term safety, we can make better choices. This helps both healthcare providers and patients.
Potential Complications of Stem Cell Treatment
Stem cell treatments are becoming more common. It’s important to know about their possible Immediate Post-Treatment Complications
Right after treatment, some people might face issues. These can be fever, headache, or fatigue from the infusion. In some cases, reactions can be more serious, like allergic responses or infections.
A study found that about 20% of patients had mild side effects after treatment. The most common were infusion-related reactions. These were usually handled well with medical care.risks. While they may help with many conditions, they are not risk-free.
| Complication Type | Frequency | Severity |
| Infusion-related reactions | 15% | Mild |
| Infections | 3% | Moderate |
| Allergic reactions | 2% | Severe |
Delayed Adverse Reactions
Some side effects can show up days or weeks later. These might include immune system reactions or unforeseen interactions with other treatments. It’s key to keep an eye on patients after treatment to catch and manage these issues.
Studies suggest that some complications can happen because of MSCs growing too much or turning into the wrong cell types. This shows why long-term checks are vital to grasp all possible risks.
Documented Cases of Harm
There have been cases where stem cell treatments caused harm. Some of these cases were very serious. This highlights the need for strict safety checks and rules.
For example, some patients got tumors or had severe immune reactions from untested stem cell treatments. These stories stress the importance of getting treatment from trusted sources and knowing the risks.
Regulatory Landscape for MSC Therapies
It’s key to know the rules for MSC therapies to use them safely and well. The rules for these therapies are complex. They involve many national and international guidelines.
FDA Stance on Stem Cell Therapy
The FDA has set rules for using MSCs in clinical trials. They stress the need for careful testing and watching for safety. These rules help make sure MSC therapies are safe and work for patients.
Key FDA Requirements:
- Investigational New Drug (IND) application for MSC-based therapies
- Rigorous preclinical and clinical testing
- Ongoing safety monitoring
International Regulatory Differences
Rules for MSC therapies vary worldwide. Some places have loose rules, while others are stricter.
Examples of International Regulatory Approaches:
- Countries with permissive regulations, allowing for more flexible use of MSC therapies
- Nations with strict regulatory frameworks, requiring thorough testing and approval
Enforcement Challenges and Actions
There are big challenges in enforcing rules, mainly with untested stem cell treatments. It’s hard for regulators to keep an eye on MSC therapy use.
Actions Taken:
- Regulatory warnings to clinics with unproven treatments
- Legal actions against those not following the rules
- Public education to tell patients about MSC therapy risks and benefits
The rules for MSC therapies are always changing. There’s a constant effort to find the right balance between new ideas and keeping patients safe.
Medical Tourism for Stem Cell Treatments
Stem cell treatments are becoming more popular. More patients are going abroad for these therapies. This has led to the growth of stem cell clinics in many countries, with varying rules.
Popular Destinations and Their Regulations
Countries with loose rules are attracting stem cell tourists. Places in Asia and Latin America are favorites because of their relaxed rules on stem cell treatments.
Regulatory frameworks differ a lot in these places. Some countries have strict rules, while others have lax regulations. This affects the quality and safety of treatments.
Risks of Seeking Treatment Abroad
Going abroad for stem cell treatments has risks. Patients might face unproven and potentially harmful therapies. The lack of strict rules can mean treatments vary in quality.
- Lack of follow-up care
- Exposure to unlicensed practitioners
- Risk of infection or complications
Patient Experiences and Outcomes
Patient experiences with stem cell treatments abroad vary. Some see good results, while others face problems. It’s key for patients to do their homework before choosing a clinic.
Knowing patient-reported outcomes is vital. It helps understand if treatments work and are safe. This info helps patients make better choices.
Clinical Trial Results: Promise vs. Reality
MSC therapies have shown great promise in clinical trials. Yet, turning these results into real-world treatments is complex. The field of stem cell therapy is growing fast, with many trials checking the safety and effectiveness of MSC-based treatments.
Major Clinical Trials and Their Findings
Many major clinical trials have shown MSC therapies working well for different conditions. For example, a study in the Journal of Translational Medicine found MSC injections helped patients with knee osteoarthritis a lot. They felt less pain and could move better.
Another trial, in Stem Cells Translational Medicine, showed MSCs helped heal wounds in patients with diabetic foot ulcers. These results highlight the promise of MSC therapies for big medical needs.
| Condition Treated | Trial Outcome | Reference |
| Knee Osteoarthritis | Significant improvement in pain and function | Journal of Translational Medicine |
| Diabetic Foot Ulcers | Enhanced wound healing | Stem Cells Translational Medicine |
Methodological Issues in MSC Research
Even with promising results, methodological issues can make it hard to understand trial data. Differences in how MSCs are sourced, prepared, and given can change trial results. Also, many trials have small sample sizes, which can make results less reliable.
Standardizing MSC therapies is key to making trial results more consistent. This will help compare results better. Work is being done to standardize how MSCs are prepared and given, which should help solve some of these issues.
Gaps Between Laboratory and Clinical Outcomes
MSC research faces a big challenge: bridging the gap between lab results and real-world treatments. MSCs have shown great promise in lab tests, but turning them into effective treatments has been slow.
Several factors contribute to this gap. Differences in MSC biology between in vitro and in vivo settings play a role. Also, human diseases are complex and hard to replicate in animal models. More research is needed to understand these gaps and find ways to translate lab results into real-world treatments.
Ethical Issues in MSC Research and Application
As MSC therapies grow, we must look closely at their ethics. The interest in MSC research has made ethics complex. We need to make sure these therapies are used safely and responsibly.
Informed Consent Challenges
Ensuring informed consent is a big ethical issue in MSC research. It’s hard to explain the risks and benefits clearly because the field is always changing. Patients need to know that many MSC treatments are experimental and their long-term effects are unknown.
MSC therapies are complex, making it hard for patients to understand their choices. Clear communication and being open are key to making sure patients are well-informed.
Exploitation of Vulnerable Patients
Another concern is the exploitation of vulnerable patients who are looking for effective treatments. Some clinics might charge high prices for unproven MSC therapies, taking advantage of patients’ desperation. It’s important for regulatory bodies to protect these patients from being exploited.
Vulnerable patients need extra care. It’s a big ethical issue to make sure they are not taken advantage of in MSC research and application.
Balancing Innovation and Patient Protection
It’s a challenge to balance innovation in MSC therapies with patient protection. We need to support research and development but also protect patients from harm. This can be done with strict regulations and ethical research practices.
Creating a culture of transparency and accountability in MSC research can help. This way, we can make sure the benefits of these therapies are realized while keeping patients safe.
Safety Concerns in Regenerative Medicine
Regenerative medicine faces many safety issues. These include problems with how it’s made, how cells are prepared, and how bad events are reported. Making sure these therapies are safe is key for their success and for patients’ trust.
Manufacturing and Quality Control Issues
Making mesenchymal stem cell (MSC) therapies is a complex task. It needs strict quality control. If the making process varies, the product’s quality can change. This might affect how safe and effective it is.
- Inconsistent cell sourcing and processing methods
- Inadequate standardization of MSC isolation and expansion techniques
- Lack of complete characterization of MSC products
Regulatory agencies are very important in checking how these therapies are made. They make sure everything follows good manufacturing practice (GMP) rules. This includes regular checks and needing detailed records of how things are made.
Cell Preparation and Delivery Risks
Getting MSC therapies ready and giving them to patients also has risks. If cells are not handled right, they might get contaminated or lose their strength.
- Risk of contamination during cell processing
- Potential for improper cell dosing or administration techniques
- Need for careful patient screening and monitoring
Doctors must be careful when giving MSC therapies. They should follow set rules to lower risks. Teaching patients about these therapies is also important. It helps them know what to expect and what might go wrong.
Monitoring and Reporting Adverse Events
It’s vital to watch and report any bad events with regenerative medicine. This work is shared by doctors and regulatory groups. They track and deal with safety issues together.
| Adverse Event Type | Reporting Requirement | Action Taken |
| Serious Adverse Events | Immediate reporting to regulatory agency | Investigation and possible removal of product |
| Non-Serious Adverse Events | Regular reporting | Review and possible change in treatment plans |
By tackling these safety issues, regenerative medicine can grow safely. This is done through strict making standards, careful cell handling, and good tracking of bad events. This way, the field can keep moving forward while keeping patients safe.
Patient-Reported Outcomes and Experiences
MSC therapies have shown mixed results, with both successes and setbacks. Patients’ experiences offer key insights into MSC therapy’s effectiveness and safety.
Success Stories and Testimonials
Many patients have seen real benefits from MSC therapies. For example, those with degenerative joint diseases have noticed improved mobility and reduced pain. This is thanks to MSCs’ ability to fight inflammation and help repair tissues.
Patients often share their hope and renewed quality of life after MSC treatments. Their stories inspire others to consider these therapies. Yet, it’s important to also look at the possible risks and benefits.
Negative Experiences and Complications
Not every patient has a positive experience with MSC therapies. Some face adverse effects, from mild reactions to serious issues. This highlights the need for careful monitoring and educating patients.
Complications can include immune reactions, infections, or unintended growths. It’s vital to document and study these issues to make MSC treatments safer.
Looking at both the good and bad outcomes from MSC therapies helps us understand them better. This balanced view is key to improving regenerative medicine and ensuring patients get the best care.
Commercialization of Stem Cell Treatments
Stem cell treatments are becoming more common, but the gap between what’s promised and what’s proven is growing. This gap raises big worries about their safety and how well they work.
Marketing Claims vs. Scientific Evidence
Many stem cell clinics make big promises about their treatments. They say their treatments can fix a lot of problems, like arthritis and brain disorders. But they don’t always back up these claims with solid science.
“Some clinics are more interested in making a profit than in providing proven, safe treatments.” This shows we need better rules and checks in the stem cell business.
- Unsubstantiated claims about treatment efficacy
- Lack of transparency regarding possible risks
- More focus on personal stories than on real research
Financial Incentives and Conflicts of Interest
Money can play a big role in stem cell treatments. Clinics and researchers might put making money first, which can hurt patient care and research.
“The lure of financial gain can sometimes cloud judgment, leading to decisions that are not in the best interest of patients.”
Money can sway:
- How research is done and results are shared
- What treatments are suggested and who gets them
- How treatments are marketed and advertised
Impact on Legitimate Research
The rise of stem cell treatments can also hurt real research. Too many untested treatments take up resources, slowing down true scientific progress.
To fix these problems, we need more openness, to stick to science, and to make sure money doesn’t harm patient care or research honesty.
The Future of Evidence-Based Regenerative Medicine
The future of regenerative medicine depends on strong, evidence-based treatments. As it grows, focusing on new research areas is key. These areas aim to make mesenchymal stem cell (MSC) therapies more effective and safe.
Emerging Research Directions
New studies show promising areas in regenerative medicine. MSCs are being explored for treating autoimmune diseases and degenerative joint disorders. They also hold promise in gene therapy and tissue engineering, opening doors to new treatments.
Improving Clinical Trial Design
To move forward, clinical trial design must get better. This means using stricter methods, like better patient choices and clear treatment plans. Improved trials will help make MSC therapies more reliable and effective.
Improving trials involves several steps:
- Setting stricter rules for who can join trials.
- Using advanced tools to track how treatments work.
- Studying the long-term effects of treatments.
Developing Consensus Standards
Creating common standards is vital for regenerative medicine. Standards for MSC use will make studies more comparable. This helps find the best ways to use MSCs in treatments.
Creating these standards needs teamwork. Researchers, doctors, regulators, and industry must work together. Together, they can unlock regenerative medicine’s full power.
Conclusion
Mesenchymal stem cells (MSCs) are becoming more common in therapy. But, we must recognize the side effects and safety issues in regenerative medicine. The good news is that MSC therapies could bring big benefits, but we also face risks.
It’s vital for patients, doctors, and scientists to understand these risks. MSC therapies are complex. We need strict safety rules and to watch for any bad effects closely.
Research should aim to improve how we test MSC therapies. We need clear standards and to solve problems with making and checking the quality of these treatments. This way, we can make sure MSC therapies are safe and effective.
In the end, being careful and based on evidence is key for MSC therapies. This careful approach will help us use MSCs to help people while keeping their safety and health first in regenerative medicine.
FAQ
What are the possible risks of stem cell therapy?
Stem cell therapy can cause side effects like pain and swelling. It can also lead to serious complications. These risks can be lessened by closely monitoring and reporting any issues.
What are the most common side effects of stem cell therapy?
Common side effects include pain, swelling, and redness at the injection site. Patients may also experience fever, headache, and fatigue. Serious issues like infection or allergic reactions can also happen.
Are there any long-term safety concerns with stem cell therapy?
Yes, there are long-term safety concerns. These include delayed reactions or secondary conditions. It’s important to keep monitoring safety to address these risks.
How do regulatory agencies ensure MSC therapies are safe?
Agencies like the FDA set guidelines for MSC therapies. They ensure these therapies are tested and approved properly. They also take action against those who don’t follow the rules.
What are the risks of getting stem cell treatment abroad?
Getting stem cell treatment abroad can be risky. You might face untested therapies and poor safety monitoring. It’s important to be cautious when considering medical tourism for these treatments.
How can I ensure safe and effective stem cell therapy?
To ensure safe therapy, consult with qualified doctors. Research the treatment and provider. Always review the informed consent documents carefully.
What is the current state of evidence-based regenerative medicine?
Regenerative medicine is growing, with new research and better clinical trials. It’s important to develop standards to guide the field. This ensures MSC therapies are safe and effective.
How can we minimize the risks of stem cell therapy?
Minimizing risks involves proper patient selection and careful planning. Ongoing safety monitoring is also key. Patients should be aware of the risks and benefits and report any issues.
What are the possible complications of stem cell treatment?
Complications can include immediate issues and delayed reactions. There have also been documented cases of harm. Understanding these risks helps mitigate them.
Are there any ethical concerns with MSC research and application?
Yes, there are ethical concerns. These include challenges with informed consent and the exploitation of vulnerable patients. It’s important to balance innovation with patient protection.
References
Baranovskii, D. S., Vorotelyak, E. A., & Makarevich, P. I. (2022). Adverse events, side effects and complications in mesenchymal stromal/stem cellbased therapy. Stem Cell Research & Therapy, 13(1), Article 479.