
Neuroblastoma is a rare and complex cancer that affects children. It starts in the adrenal glands, neck, chest, or spinal cord. This disease is hard to treat because it doesn’t respond well to many therapies Neuroblastoma treatment.
Fighting neuroblastoma is tough. It’s because of its aggressive nature and how it doesn’t react well to common treatments.
Key Takeaways
- Neuroblastoma is a complex and rare childhood cancer.
- The disease often resists conventional treatment methods.
- Understanding the challenges in treating neuroblastoma is key.
- Research is ongoing to improve treatment outcomes.
- Support for families affected by neuroblastoma is vital.
The Nature and Complexity of Neuroblastoma
Neuroblastoma starts from immature nerve cells in the sympathetic nervous system. It’s a cancer that mainly hits kids. Its origin in the sympathetic nervous system makes it quite complex.
Origin and Development in the Sympathetic Nervous System
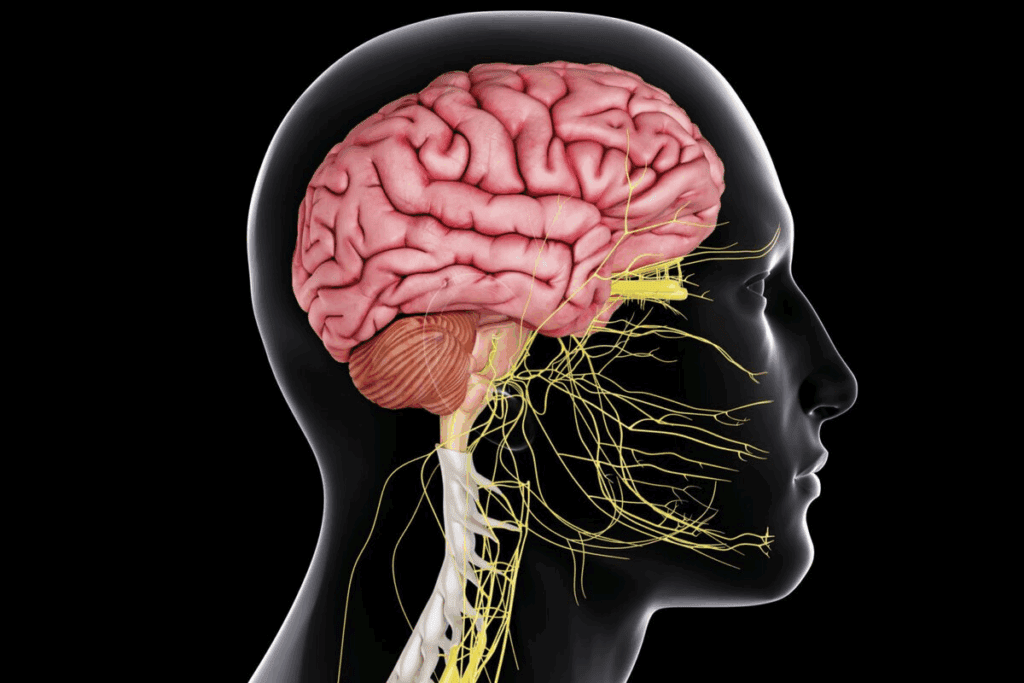
Neuroblastoma comes from neuroblasts, which are young nerve cells in the sympathetic nervous system. This system is part of the autonomic nervous system. It controls things we do without thinking.
Neuroblasts should grow into sympathetic neurons. But in neuroblastoma, they keep growing without stopping.
Neuroblastoma can start anywhere from the neck to the pelvis. This makes it hard to diagnose and treat.
Unique Characteristics of Pediatric Solid Tumors
Neuroblastoma is a type of solid tumor found in kids. Solid tumors are different from liquid ones like leukemia. They can be very aggressive or sometimes just go away on their own.
Another thing that makes neuroblastoma tough is how different each tumor can be. They can have different genes, act differently, and react to treatment in many ways. This makes it hard to find a treatment that works for everyone.
| Characteristics | Description |
| Origin | Develops from immature nerve cells (neuroblasts) in the sympathetic nervous system |
| Location | Can occur anywhere along the sympathetic nervous system (neck to pelvis) |
| Tumor Behavior | Can be aggressive or spontaneously regress |
| Heterogeneity | Significant variability in genetic makeup, behavior, and treatment response |
Genetic and Molecular Complexities

Understanding the genetic complexities of neuroblastoma is key to finding effective treatments. Neuroblastoma mainly affects children and has a wide range of genetic and molecular features.
MYCN Amplification and Its Impact on Treatment Resistance
MYCN amplification makes neuroblastoma more aggressive and harder to treat. This genetic change is linked to more advanced disease and a worse outlook. Studies have found that MYCN amplification makes tumors grow faster and resist standard treatments.
ALK Mutations and Other Genetic Alterations
ALK mutations are also important in neuroblastoma. These mutations can turn on the ALK tyrosine kinase, helping tumors grow and resist treatment. Finding these mutations is vital for creating targeted therapies that can help patients more.
Tumor Heterogeneity and Clonal Evolution
Neuroblastoma tumors are often very different, with various clones having unique genetic traits. This diversity can cause clonal evolution, where aggressive clones grow and spread, making treatment harder and leading to disease coming back.
The complex mix of these genetic factors shows we need a deep understanding of neuroblastoma’s genetics and molecular makeup. By exploring these complexities, we can create more tailored and effective treatments to better help patients.
Challenges in Early Detection and Diagnosis
Finding neuroblastoma early is hard. This is because it doesn’t show clear signs at first. Also, today’s tests aren’t perfect.
Neuroblastoma can display various symptoms that are similar to other childhood illnesses. These non-specific symptoms make it hard to spot neuroblastoma right away.
Non-specific Symptoms Leading to Delayed Diagnosis
Neuroblastoma symptoms include belly pain, swelling, or a lump. It can also cause fever, weight loss, and tiredness. These signs are common in many illnesses, leading to a late diagnosis.
For example, a child with belly pain might first be thought to have a stomach problem. This can mean neuroblastoma is missed. Early detection is key because it affects how well the treatment works.
Biomarker Limitations for Early Detection
Today’s biomarkers for neuroblastoma, like urine tests, aren’t always accurate. They help but can’t catch it early enough.
Scientists are working on better biomarkers. They hope these will find neuroblastoma sooner. Until then, doctors use a mix of symptoms, scans, and lab tests to diagnose.
The Staging Dilemma in Neuroblastoma
Understanding the staging dilemma in neuroblastoma is key for effective treatment planning. Neuroblastoma staging is vital for knowing the prognosis and guiding treatment. But, staging this disease is complex.
The International Neuroblastoma Risk Group (INRG) staging system is widely used. It classifies neuroblastoma based on disease extent and image-defined risk factors. Yet, it has limitations, mainly in distinguishing between high-risk and low-risk classifications.
High-Risk vs. Low-Risk Classification Challenges
Accurately classifying patients as high-risk or low-risk is a major challenge. High-risk neuroblastoma has a poor prognosis and needs intense treatment. Low-risk disease often has a better outcome with less aggressive therapy.
The line between high-risk and low-risk neuroblastoma is not always clear. Age, tumor biology, and metastatic disease influence risk classification. For example, MYCN amplification can increase a patient’s risk status.
| Risk Category | Characteristics | Treatment Approach |
| Low-Risk | Localized disease, favorable biology | Surgery, observation |
| High-Risk | Advanced disease, MYCN amplification | Intensive chemotherapy, radiation, immunotherapy |
Treatment Complexity in Stage 4 Neuroblastoma
Stage 4 neuroblastoma is a challenging subset of the disease, with distant metastasis. Treatment for stage 4 neuroblastoma is complex. It often includes intensive chemotherapy, radiation therapy, and immunotherapy.
“The treatment of stage 4 neuroblastoma requires a coordinated effort from a multidisciplinary team, including pediatric oncologists, surgeons, and radiation therapists, to tailor therapy to the individual patient’s needs.”
The complexity of treating stage 4 neuroblastoma is increased by the disease’s heterogeneity. Some patients may have refractory disease or develop resistance to therapy. This requires innovative treatment strategies.
In conclusion, the staging dilemma in neuroblastoma highlights the need for continued research. We need more accurate and effective staging systems. By improving our understanding of neuroblastoma biology and developing personalized treatment approaches, we can work towards better outcomes for patients with this challenging disease.
Neuroblastoma Treatment: Current Approaches and Limitations
Neuroblastoma treatment is getting better, thanks to new methods. Doctors now use a mix of treatments like surgery, chemotherapy, and radiation. They also use immunotherapy to fight the disease.
The Necessity for Multimodal Treatment Strategies
Using many treatments at once is key in fighting neuroblastoma. Surgery helps remove the main tumor. Chemotherapyand radiation therapy target the tumor and any cancer that has spread. Immunotherapy, like anti-GD2 antibodies, helps high-risk patients a lot.
This mix of treatments lets doctors plan care that fits each patient. But, it makes treatment planning harder and can cause more side effects.
Balancing Efficacy and Long-term Toxicity in Children
One big challenge is finding treatments that work well without harming kids too much. Chemotherapy and radiation therapy can hurt a child’s growth and life quality.
Doctors must think carefully about each treatment’s benefits and risks. Precision medicine aims to use treatments that match the tumor’s genetic makeup. This could lower side effects while keeping treatment effective.
By improving treatment plans and adding new therapies, we aim to help kids with neuroblastoma. We want to make sure they get better without lasting harm from treatment.
Surgical Intervention Challenges
Surgery for neuroblastoma is very hard because of the tumor’s tricky location. It needs a team of very skilled surgeons.
Anatomical Complexities and Tumor Location Issues
Neuroblastoma can grow in the adrenal glands, neck, chest, or spinal cord. These places have many important structures. The closeness to vital blood vessels, nerves, and organs makes surgery hard.
Incomplete Resection and Its Consequences
Not fully removing the tumor can cause it to come back. This shows how vital it is to remove all of the tumor. But, there’s a risk of complications during surgery.
Some big challenges in removing the tumor include:
- Tumors that are deeply embedded in vital structures
- Tumors with extensive vascular involvement
- Tumors located in areas that are difficult to access surgically
To tackle these issues, surgeons use advanced imaging and monitoring during surgery. This helps them remove the tumor fully while reducing risks.
Chemotherapy Resistance Mechanisms
Understanding how chemotherapy resistance works is key to better treating neuroblastoma. Chemotherapy is a mainstay in fighting neuroblastoma. But, resistance often limits its success.
Resistance to chemotherapy in neuroblastoma comes from different sources. We’ll look at how intrinsic and acquired resistance work. We’ll also explore the role of drug efflux pumps and detox systems.
Intrinsic and Acquired Drug Resistance Pathways
Some neuroblastoma cells are naturally resistant to chemotherapy. This is because of their genetic makeup.
Other cells develop resistance over time. This happens as they adapt to the effects of chemotherapy. Changes in their genes and how their genes are turned on or off play a part.
- Genetic mutations that alter drug targets
- Activation of survival pathways
- Enhanced DNA repair mechanisms
Drug Efflux Pumps and Detoxification Systems
Drug efflux pumps, like P-glycoprotein (P-gp), are a big part of resistance. They push chemotherapy drugs out of cells, making them less effective.
Neuroblastoma cells also boost detox systems. These systems, like glutathione S-transferase (GST) enzymes, can neutralize chemotherapy drugs.
The complex ways resistance develops show we need a broad approach to beat it in neuroblastoma. By grasping these mechanisms, we can craft more effective treatments.
Radiation Therapy Limitations in Pediatric Patients
Radiation therapy is key in fighting cancers like neuroblastoma. But, it’s tough to use it on kids. We must balance treating the tumor and avoiding long-term harm.
Growth and Development Concerns
One big worry is how radiation affects kids’ growth. “Radiation can harm bone growth plates, causing problems,” says a pediatric oncologist. It can also hurt the development of nearby tissues and organs.
This can lead to lasting health issues. It can make survivors’ lives harder.
Younger kids are more at risk. Using radiation on very young children can cause serious problems. These include bone deformities and organ damage.
Secondary Malignancy Risks
Another big issue is the chance of getting new cancers. Radiation can increase this risk. This is a big worry for kids, who may live a long time after treatment.
These new cancers can show up years later. So, it’s very important to keep an eye on kids who have had radiation therapy. We must think carefully about the benefits and risks of using radiation.
“The risk of secondary cancers following radiation therapy is a critical consideration in the treatment of pediatric neuroblastoma, necessitating careful planning and follow-up,” notes a leading oncology researcher.
We’re always looking for ways to lower these risks. New techniques, like proton therapy, might help protect healthy tissues from radiation.
The Blood-Brain Barrier and CNS Involvement
The blood-brain barrier is key to brain health but makes treating neuroblastoma hard. It’s made of tight cells that block most molecules, including drugs. This keeps the brain safe from harmful substances.
Challenges in Drug Delivery to CNS Metastases
Getting drugs to CNS metastases in neuroblastoma is tough. The blood-brain barrier stops many drugs from reaching the brain tumors. This means treatments often fail to fully treat CNS metastases, leading to disease growth.
Creating ways to get drugs past the blood-brain barrier is a big challenge. Neuroblastoma’s complexity and the CNS’s protective nature require new drug delivery methods.
Strategies to Overcome the Blood-Brain Barrier
Researchers are working on several ways to beat the blood-brain barrier. They’re making drugs that can get through, using methods to open the barrier, and targeting specific CNS areas.
One exciting idea is nanoparticles that can get into the brain. These tiny particles are made to carry drugs right to CNS metastases. This could lead to better treatment results.
| Strategy | Description | Potential Benefits |
| Drug Development | Creating drugs that can cross the blood-brain barrier | Improved efficacy in treating CNS metastases |
| Temporary Barrier Disruption | Using methods to temporarily open the blood-brain barrier | Enhanced drug delivery to the CNS |
| Nanoparticle Technology | Employing nanoparticles to target CNS metastases | Targeted therapy with reduced side effects |
By tackling the blood-brain barrier’s challenges and finding new solutions, we can help more neuroblastoma patients. This includes those with CNS involvement.
Metastatic Spread and Microenvironment Interactions
It’s key to know how neuroblastoma cells and their surroundings interact. This knowledge helps in finding better treatments. Neuroblastoma spreads quickly and affects the tissue around it, impacting how the disease grows and how well treatments work.
Bone Marrow Infiltration Mechanisms
Neuroblastoma cells often move into the bone marrow, which is a bad sign. The bone marrow acts as a safe place for these cells, making treatments less effective. Bone marrow infiltration involves complex interactions between tumor cells and the bone marrow stroma, helping the cells survive and resist treatment.
Tumor Microenvironment and Treatment Resistance
The area around the tumor greatly affects how neuroblastoma cells behave and react to treatments. The microenvironment can make cells resistant to chemotherapy and targeted therapies by secreting substances that help them live longer.
- The tumor microenvironment can limit the effectiveness of chemotherapy.
- Cancer-associated fibroblasts and immune cells contribute to treatment resistance.
- Targeting the tumor microenvironment is a promising therapeutic strategy.
Angiogenesis and Vascular Targeting Challenges
Angiogenesis, or making new blood vessels, is a key feature of cancer, including neuroblastoma. Targeting angiogenesis is a possible treatment, but it’s hard because of the complex nature of blood vessels and the ways cells can resist treatment.
“The inhibition of angiogenesis has been shown to be effective in some cancers, but its application in neuroblastoma requires further investigation.”
Mechanisms of Tumor Recurrence
Tumor recurrence in neuroblastoma often comes from small groups of tumor cells that survive treatment. These cells can cause the tumor to come back. It’s important to understand how this happens to find ways to stop it.
Detection and Management of Minimal Residual Disease
Finding small groups of tumor cells is hard and needs very sensitive tools. Advanced molecular techniques and imaging are being looked at to find these cells. To manage these cells, doctors use targeted and immunotherapies to get rid of them.
We’re getting closer to understanding how neuroblastoma cells and their environment interact. More research is needed to turn this knowledge into better treatments for metastatic neuroblastoma and to prevent it from coming back.
Immunotherapy Challenges in Neuroblastoma
Immunotherapy in treating neuroblastoma shows great promise but faces big hurdles. It uses the immune system to fight cancer, which is a strong tool against neuroblastoma. But, it’s not without its challenges.
Limitations of Anti-GD2 Antibody Therapy
Anti-GD2 antibody therapy is a big step forward in treating neuroblastoma. It targets the GD2 antigen on neuroblastoma cells. Yet, it comes with severe side effects like pain and neuropathy. Not all patients respond, and some develop resistance over time.
To improve anti-GD2 therapy, researchers are looking at combining it with other treatments. They also want to use it with chemotherapy. More research is needed to make it safer and more effective.
CAR T-Cell Therapy Development Hurdles
CAR T-cell therapy is another promising approach for neuroblastoma. It genetically modifies T cells to attack cancer cells. While it’s worked well in some leukemias, it’s harder to use in solid tumors like neuroblastoma.
One big challenge is getting CAR T cells to reach and attack tumor cells. The process of making CAR T cells is also complex and expensive. This makes it hard for many patients to get this treatment.
| Challenge | Description | Potential Solution |
| Targeting Tumor Cells | CAR T cells face difficulty penetrating solid tumors. | Improving CAR T-cell design for better tumor infiltration. |
| Manufacturing Complexity | The process of creating CAR T cells is complex and costly. | Streamlining manufacturing processes and reducing costs. |
Immune Evasion Mechanisms in Neuroblastoma
Neuroblastoma cells can avoid the immune system in many ways. They can express molecules that stop T cells from working. They also create an environment that stops immune cells from attacking.
To beat these challenges, researchers are looking at ways to block these immune stops. They’re also working on changing the tumor environment. This includes using checkpoint inhibitors and other treatments to boost the immune response.
By tackling the challenges of immunotherapy in neuroblastoma, we can develop better treatments. Ongoing research and clinical trials are key. They offer hope for better outcomes for patients with neuroblastoma.
Targeted Therapy Development Obstacles
Creating targeted therapies for neuroblastoma is tough. We face big challenges in making treatments that are safe and fit the needs of kids.
Pediatric-Specific Drug Development Barriers
One big challenge is making drugs for kids. It’s hard because of several reasons:
- Limited patient populations, making it hard to do big clinical trials.
- Differences in pediatric physiology compared to adults, affecting how drugs work and how safe they are.
- Ethical considerations in doing clinical trials on kids, needing to weigh risks and benefits carefully.
These issues make it harder and more complex to develop drugs for pediatric neuroblastoma.
Resistance to Molecular Targeted Therapies
Another big challenge is when tumors resist targeted therapies. Neuroblastoma tumors can resist in many ways, like:
- Genetic mutations that change the drug target, making it less effective.
- Activation of alternative signaling pathways, letting the tumor avoid the targeted pathway.
- Enhanced drug efflux, lowering the drug’s concentration inside the tumor cells.
It’s key to understand these resistance ways to find new ways to beat them and make targeted therapies work better.
We’re pushing forward with research and new therapy development. Our goal is to better treat pediatric neuroblastoma patients.
Clinical Trial Challenges in Pediatric Oncology
Running clinical trials for kids with cancer is tough. It’s hard to find enough kids for these studies. This makes it hard to find new treatments that really help.
Limited Patient Populations and Ethical Considerations
One big problem is finding enough kids for trials. Kids’ cancers are rare, so it’s hard to get enough kids for studies. It’s also very important to make sure kids are safe while trying new treatments.
Using placebos in trials for kids is tricky. If there’s a good treatment already, it’s hard to not give it to kids. This makes it tough to design fair trials.
Regulatory and Pharmaceutical Industry Challenges
Rules for trials on kids vary by country. This makes it hard to do trials that work in many places. It’s also hard for drug companies to make drugs for kids because of the small market and high costs.
Here’s a table showing some of the big rules for trials on kids with cancer:
| Regulatory Aspect | Description | Challenge |
| Informed Consent | Getting parents to agree for their kids to join trials. | It’s hard to make sure consent is real and free. |
| Ethics Review | Checking if trials are okay to do. | Rules for this vary a lot. |
| Regulatory Approval | Getting the okay to start trials. | Rules are different everywhere. |
We need to work together to solve these problems. By teaming up, we can find better treatments for kids with cancer. We can make sure trials are safe and work well.
Conclusion: Future Directions in Overcoming Neuroblastoma Treatment Resistance
Treating neuroblastoma is tough because of its complex biology and treatment resistance. We’ve talked about the main hurdles. These include genetic and molecular complexities, early detection and diagnosis challenges, and current treatment limits.
Research and innovation are key to beating these challenges. New targeted therapies, better immunotherapy, and understanding the tumor environment are on the horizon. We’re seeing big steps in neuroblastoma research, focusing on precision medicine and tailored treatments.
Neuroblastoma treatment is set to get a boost from teamwork between researchers, doctors, and industry. New approaches like CAR T-cell therapy and ways to get past the blood-brain barrier could help. As we keep exploring, understanding neuroblastoma’s biology is vital for better treatments and care.
FAQ
What is neuroblastoma and how does it develop?
Neuroblastoma is a cancer that starts in the nervous system. It mainly affects kids. It comes from immature nerve cells, but we don’t know exactly why it happens.
What are the challenges in diagnosing neuroblastoma?
Finding neuroblastoma can be hard because its symptoms are not clear. These symptoms can look like other diseases. Also, current tests are not perfect, making it tough to catch early.
How is neuroblastoma staged, and what are the challenges in staging?
Doctors use a system to see how far the cancer has spread. Stage 4 is the worst. But, it’s hard to tell if it’s a high-risk or low-risk case, which is tricky.
What are the current treatment approaches for neuroblastoma?
Doctors use many ways to treat neuroblastoma. This includes surgery, chemo, radiation, and immunotherapy. The big challenge is finding treatments that work well without harming kids too much.
Why is chemotherapy resistance a significant issue in neuroblastoma treatment?
Chemotherapy doesn’t always work because of several reasons. These include the cancer cells’ ability to resist drugs and get rid of them. This can make treatment fail and the cancer come back.
What are the limitations of radiation therapy in pediatric patients with neuroblastoma?
Radiation can help fight neuroblastoma. But, it can also hurt kids’ growth and increase the risk of getting another cancer later.
How does the blood-brain barrier impact neuroblastoma treatment?
The blood-brain barrier makes it hard to get drugs to cancer in the brain. This is a big problem for treating neuroblastoma that has spread to the brain. Scientists are working on ways to get past this barrier.
What role does the tumor microenvironment play in neuroblastoma treatment resistance?
The area around the tumor, like bone marrow and blood vessels, can help the cancer grow and resist treatment. This makes it harder to fight the cancer.
What are the challenges in developing effective immunotherapies for neuroblastoma?
Making immunotherapies for neuroblastoma is tough. Problems include the limits of anti-GD2 antibodies and CAR T-cells. Also, the cancer can find ways to avoid the immune system.
What obstacles exist in developing targeted therapies for neuroblastoma?
Creating targeted treatments for neuroblastoma is hard. This is because of the challenges of making drugs for kids and the risk of the cancer becoming resistant to these treatments.
What challenges are faced in conducting clinical trials for pediatric oncology?
Doing clinical trials for kids with cancer is hard. There are not enough patients, and there are rules to follow. This makes it hard to find new treatments.
What future directions hold promise for improving neuroblastoma treatment outcomes?
There is hope for better treatments for neuroblastoma. Research is ongoing in immunotherapy, targeted therapy, and understanding the cancer’s genetics. These areas show promise for better results.
Reference
- Pinto, N. R., Applebaum, M. A., Volchenboum, S. L., Matthay, K. K., London, W. B., Ambros, P. F., & Cohn, S. L. (2015). Advances in risk classification and treatment strategies for neuroblastoma. Journal of Clinical Oncology, 33(27), 3008–3017. https://pubmed.ncbi.nlm.nih.gov/26304901/



































