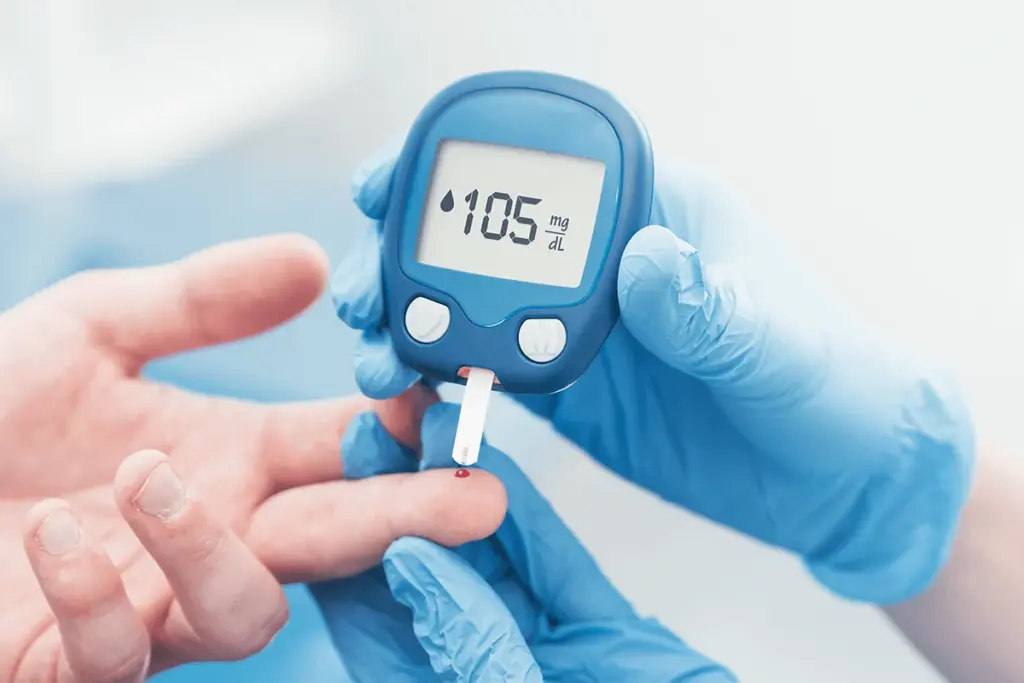
Diabetes mellitus represents a group of complex metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or a combination of both.
Send us all your questions or requests, and our expert team will assist you.
Diabetes mellitus represents a group of complex metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or a combination of both. This condition fundamentally alters how the body utilizes blood sugar, which is a vital source of energy for the cells that make up muscles and tissues and is the primary fuel source for the brain. Under normal physiological conditions, glucose levels are tightly regulated by insulin, a hormone produced by the pancreas. When this regulatory system falters, glucose accumulates in the bloodstream rather than being transported into cells. The long-term implications of this systemic dysregulation are profound, potentially leading to the failure of various organs, specifically the eyes, kidneys, nerves, heart, and blood vessels.
The scope of diabetes is not limited to a single mechanism but involves a spectrum of metabolic challenges. The disease burden is significant globally, affecting individuals of all ages and demographics. Understanding diabetes requires a comprehensive view of the interplay between genetics, environmental factors, and lifestyle behaviors. It is a chronic condition that demands lifelong management, yet with modern medical advancements, individuals can lead full and active lives. The definition of diabetes has evolved from a simple disorder of sugar to a multi-faceted cardiovascular and metabolic syndrome that requires a holistic approach to care. This section explores the fundamental biology, distinct classifications, and the physiological underpinnings that define this widespread health challenge.

The human body operates on a delicate balance of energy intake and expenditure, with glucose serving as the central currency of this energy exchange. The pancreas, a gland located behind the stomach, plays the starring role in this process. Within the pancreas are specialized regions called the islets of Langerhans, which house beta cells and alpha cells. These cells function as the body’s internal thermostat for blood sugar, constantly sensing glucose levels and releasing hormones to maintain equilibrium. When this system works correctly, blood sugar remains within a narrow, healthy range regardless of food intake or physical activity.
However, in diabetes, this homeostatic loop is broken. The breakdown can occur at the point of production, where the pancreas fails to generate sufficient insulin, or at the cellular level, where the body’s tissues fail to respond to the insulin that is present. Understanding the specific roles of the primary hormones involved is essential for grasping the mechanics of the disease.
Insulin is effectively the master key that unlocks the body’s cells to allow glucose to enter and be used for energy. When a person consumes carbohydrates, they are broken down into glucose, which enters the bloodstream. The rise in blood glucose signals the beta cells in the pancreas to release insulin. This hormone travels through the blood to insulin receptors on the surface of cells, particularly in muscle, fat, and liver tissue. Once attached, insulin facilitates the transport of glucose from the blood into the cell. Furthermore, insulin signals the liver to store excess glucose as glycogen for later use. Without adequate insulin, sugar remains trapped in the bloodstream, starving the cells of energy and causing the toxic effects of hyperglycemia.
Glucagon acts as the counterbalance to insulin, ensuring that blood sugar does not drop dangerously low, a state known as hypoglycemia. Produced by the alpha cells in the pancreas, glucagon is released when blood glucose levels fall, such as during fasting, between meals, or during intense exercise. Its primary function is to signal the liver to convert stored glycogen back into glucose and release it into the bloodstream. This counter-regulatory mechanism ensures that the brain and vital organs receive a steady supply of fuel even when food is not being consumed. In diabetes, the communication between insulin and glucagon is often disrupted, leading to erratic blood sugar fluctuations.

Type 1 diabetes is an autoimmune condition where the body’s immune system mistakenly attacks and destroys the insulin-producing beta cells in the pancreas. This process is often irreversible and results in a near-total or total deficiency of insulin. Consequently, individuals with Type 1 diabetes are dependent on exogenous insulin for survival. This form of diabetes was historically termed juvenile diabetes because it is frequently diagnosed in children and young adults, but it can manifest at any age.
The exact cause of the immune system’s error remains a subject of intense research, though it is believed to be a combination of genetic susceptibility and an environmental trigger, such as a viral infection. Unlike Type 2 diabetes, Type 1 is not linked to lifestyle factors such as diet or physical inactivity. The onset is typically rapid and dramatic. Clinically, it is distinguished by the presence of autoantibodies in the blood that target pancreatic tissues. Because the body cannot produce insulin, glucose accumulates rapidly in the blood, and the body turns to burning fat for fuel, producing ketones, which can lead to a dangerous condition called diabetic ketoacidosis.

Type 2 diabetes is the most common form of the disease, accounting for the vast majority of cases worldwide. It is characterized by a dual defect: the body’s cells become resistant to the action of insulin, and the pancreas gradually loses the ability to produce enough insulin to overcome this resistance. Unlike Type 1, the onset of Type 2 diabetes is often insidious and gradual, developing over years. It is strongly associated with age, obesity, family history, and physical inactivity, although genetic factors also play a significant role.
The progression of Type 2 diabetes involves a continuum of metabolic changes. Initially, the pancreas compensates for insulin resistance by working overtime to produce more insulin. This state of hyperinsulinemia can maintain normal blood sugar levels for a time. Eventually, the beta cells become exhausted and can no longer keep up with the demand, leading to rising blood sugar levels and the clinical diagnosis of diabetes.
Insulin resistance is a condition where the body’s cells in the muscles, fat, and liver do not respond effectively to insulin. Imagine a lock that has become rusty; the key (insulin) enters, but it requires much more effort to turn and open the door. Because the cells do not easily absorb glucose, sugar builds up in the blood. The liver also contributes to the problem by continuing to release glucose into the bloodstream even when levels are already high, failing to receive the signal from insulin to stop production. This resistance is often driven by excess adipose tissue, particularly visceral fat around the abdomen, which releases inflammatory substances that interfere with insulin signaling.
While insulin resistance is a primary driver, Type 2 diabetes does not develop unless there is also beta-cell dysfunction. In a healthy individual, if insulin resistance develops, the pancreas simply produces more insulin to compensate. In those who develop Type 2 diabetes, the beta cells are unable to sustain this increased output. Over time, the chronic stress of overproduction, combined with the toxic effects of high glucose (glucotoxicity) and high fatty acids (lipotoxicity), leads to the structural damage and programmed cell death of beta cells. This progressive loss of insulin secretory capacity is why Type 2 diabetes is considered a progressive disease that may require escalating treatment over time.
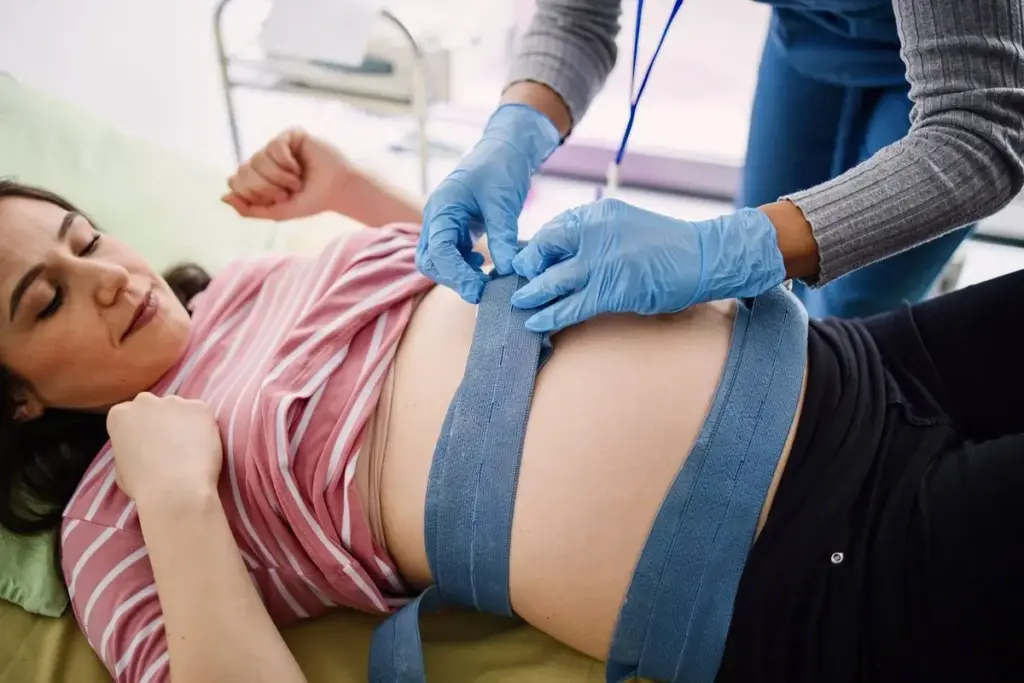
Gestational diabetes mellitus is a form of diabetes that is first diagnosed during pregnancy. It occurs when the body cannot make enough insulin to meet the extra needs of pregnancy. During gestation, the placenta produces hormones to help the baby grow, but these hormones also cause insulin resistance in the mother’s body. Usually, the mother’s pancreas produces more insulin to overcome this resistance. When the pancreas cannot keep up, glucose levels rise, resulting in gestational diabetes.
While blood sugar levels typically return to normal after childbirth, women who have had gestational diabetes are at a significantly higher risk of developing Type 2 diabetes later in life. Furthermore, the condition poses risks to the infant, including high birth weight (macrosomia), low blood sugar at birth, and a higher likelihood of developing obesity and Type 2 diabetes in their own future. Management involves rigorous blood sugar monitoring, dietary changes, and sometimes medication to ensure the health of both mother and child.
Prediabetes is a precursor stage where blood glucose levels are higher than normal but not yet high enough to be classified as Type 2 diabetes. It serves as a critical warning sign. Without lifestyle intervention, a significant portion of people with prediabetes will progress to overt Type 2 diabetes. This stage is often asymptomatic, meaning damage to the heart and circulatory system may occur before the patient is aware of the condition.
Prediabetes is frequently part of a broader cluster of conditions known as metabolic syndrome. This syndrome is not a specific disease but a collection of risk factors that occur together and increase the risk of heart disease, stroke, and type 2 diabetes. These factors include increased blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol or triglyceride levels. Addressing prediabetes involves the same strategies used to manage diabetes itself, primarily focused on weight reduction and increased physical activity to improve insulin sensitivity.
Beyond the primary classifications, there are several specific types of diabetes that arise from different causes. These include Monogenic Diabetes, which results from mutations in a single gene and is often inherited. Examples include Neonatal Diabetes Mellitus and Maturity-Onset Diabetes of the Young (MODY). These forms are rare and can be misdiagnosed as Type 1 or Type 2, leading to inappropriate treatment.
Other types include secondary diabetes caused by other conditions or exposures. For instance, diseases of the exocrine pancreas, such as cystic fibrosis or chronic pancreatitis, can damage beta cells, leading to pancreatogenic diabetes. Additionally, certain medications, particularly corticosteroids used for inflammation, can induce diabetes or significantly worsen blood sugar control in susceptible individuals. Identifying the correct type is crucial because the treatment strategies may differ significantly from standard protocols.

Send us all your questions or requests, and our expert team will assist you.
The cause depends on the type. Type 1 is caused by an autoimmune reaction destroying insulin cells, while Type 2 is caused by a combination of genetic factors and lifestyle choices leading to insulin resistance.
Currently, there is no permanent cure for diabetes. However, it can be effectively managed, and in some cases of Type 2, it can go into remission where blood sugar levels return to a healthy range without medication.
Eating sugar does not directly cause Type 1 diabetes. For Type 2, a diet high in sugar and calories can lead to weight gain, which is a primary risk factor for developing the condition.
Type 1 is an autoimmune condition where the body produces no insulin and usually appears early in life. Type 2 involves the body not using insulin well and is often linked to aging and lifestyle.
Insulin acts as a key that allows glucose to enter cells from the bloodstream. It lowers blood sugar levels and enables the body to use sugar for energy or store it for later.


Leave your phone number and our medical team will call you back to discuss your healthcare needs and answer all your questions.
Your Comparison List (you must select at least 2 packages)