Last Updated on December 3, 2025 by Bilal Hasdemir
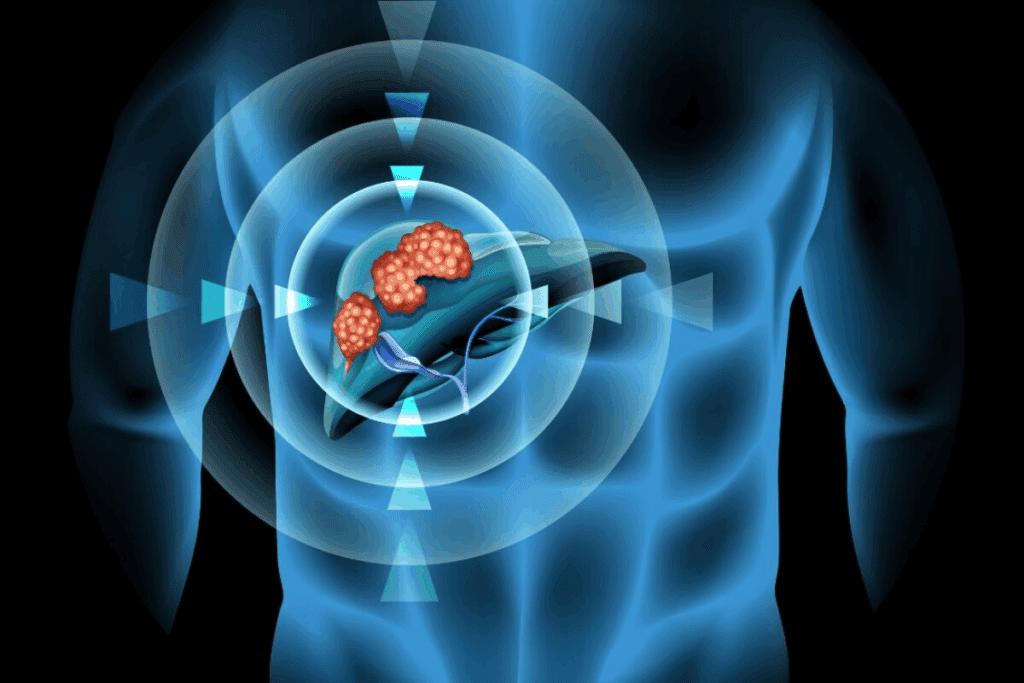
Hepatoblastoma is a rare and aggressive liver tumor mainly found in children. A significant number of cases are linked to genetic predisposition. This shows how vital it is to grasp the syndromes associated with it.
Studies reveal that some genetic syndromes raise the risk of getting hepatoblastoma, a type of childhood liver cancer. We will dive into these syndromes, their genetic roots, and what they mean for patients and their families.
By grasping the connection between genetic predisposition and hepatoblastoma, we can better spot and treat it early.
Key Takeaways
- Genetic syndromes play a significant role in the development of hepatoblastoma.
- Understanding these syndromes is key for early detection and management.
- Hepatoblastoma is a rare liver cancer that mainly hits children.
- Certain genetic predispositions up the risk of getting hepatoblastoma.
- Early detection and management strategies can get better by knowing the genetic link.
Understanding Hepatoblastoma: A Primer
Hepatoblastoma is a rare liver cancer in kids. It’s a big worry for doctors who treat children with cancer. To understand its link to genetic syndromes, we must first learn about this rare cancer.
Definition and Incidence of Hepatoblastoma
Hepatoblastoma is a cancer of the liver found mainly in kids under three. It’s a rare cancer, making up about 1% of all cancers in kids. It happens in about 1.2 cases per million kids under 15, mostly in those under three.
Pathophysiology and Histological Subtypes
Hepatoblastoma happens when liver cells grow too much and form tumors. It has different types, like epithelial, mixed, and small cell undifferentiated. Knowing these types helps doctors plan treatment and predict outcomes.
The epithelial type is split into fetal and embryonal, with fetal being more hopeful. The mixed type has both epithelial and mesenchymal parts, like bone or cartilage.
Risk Factors and Genetic Predisposition
Several things increase the risk of getting hepatoblastoma. These include being very small at birth and certain genetic syndromes. Genes play a big role, with conditions like Beckwith-Wiedemann Syndrome (BWS) and Familial Adenomatous Polyposis (FAP) raising the risk.
Knowing these risk factors and genetic links is key to catching and treating hepatoblastoma early. We’ll explore these genetic syndromes more in the next sections.
The Genetic Basis of Hepatoblastoma-associated Syndromes
Understanding the genetics behind hepatoblastoma-associated syndromes is key for treatment. Hepatoblastoma is a rare liver cancer mainly in kids. It’s linked to genetic syndromes. These syndromes come from complex genetic changes that help hepatoblastoma grow.
Chromosomal Abnormalities and Copy Number Variations
Chromosomal changes and copy number variations (CNVs) are big in these syndromes. They mess with genes that control cell growth. For example, trisomy 18, or Edwards syndrome, raises the risk of hepatoblastoma.
Changes in genes like APC and GPC3 are linked to FAP and Simpson-Golabi-Behmel syndrome. These changes can make genes work too much or too little. This can lead to tumors. Knowing these changes helps find at-risk people and set up better screening.
Germline vs. Somatic Mutations
Germline and somatic mutations both play a part in these syndromes. Germline mutations are passed down and are in every cell. Somatic mutations happen in certain cells.
Germline mutations in TP53 and APC genes raise the risk of hepatoblastoma. Somatic mutations in important genes can also lead to tumors. Knowing the difference helps with genetic counseling and screening.
Epigenetic Mechanisms in Hepatoblastoma Development
Epigenetic changes, like DNA methylation and histone modification, also play a role. These changes affect how genes work without changing the DNA. For instance, wrong methylation can silence tumor suppressor genes, helping tumors grow.
It’s important to understand how genetics and epigenetics work together in these syndromes. More research could lead to new treatments and better care for patients.
Beckwith-Wiedemann Syndrome: The Most Common Association
Beckwith-Wiedemann Syndrome (BWS) is a rare genetic disorder. It is known for causing overgrowth and an increased risk of childhood tumors. This includes hepatoblastoma.
Clinical Features and Diagnostic Criteria
BWS is diagnosed by looking at several signs. These include being very big, having a big tongue, and certain birth defects. Genetic tests also help confirm the diagnosis. Early diagnosis is key to managing risks, like hepatoblastoma.
“The signs of BWS can differ a lot from person to person,” experts say. A detailed check-up is needed for a correct diagnosis.
Molecular Subtypes and Imprinting Disorders
BWS is linked to changes on chromosome 11p15.5. These changes affect how genes work. Knowing about these changes helps predict tumor risk and plan care.
- Imprinting center 1 (IC1) abnormalities
- Imprinting center 2 (IC2) abnormalities
- Uniparental disomy of chromosome 11
- CDKN1C mutations
These genetic changes can make genes that help cells grow too much. They can also silence genes that stop tumors. This increases the risk of hepatoblastoma.
Hepatoblastoma Risk Stratification in BWS Patients
For BWS patients, figuring out the risk of hepatoblastoma is important. It involves looking at symptoms, genetic tests, and regular check-ups. Alpha-fetoprotein (AFP) tests and ultrasound scans are key parts of this.
By knowing who is at higher risk, we can plan better care. This can lead to better outcomes for BWS patients.
“Early detection and a team effort are the best ways to manage hepatoblastoma risk in BWS patients.”
Familial Adenomatous Polyposis (FAP) and Hepatoblastoma
Familial Adenomatous Polyposis (FAP) is linked to colorectal cancer. It also raises the risk of hepatoblastoma. FAP causes many polyps in the colon and rectum, leading to cancer and other tumors.
APC Gene Mutations and Cancer Predisposition
FAP comes from APC gene mutations. These mutations cause many polyps in the colon and rectum. This greatly increases the risk of colorectal cancer. APC mutations also raise the risk of other cancers, like hepatoblastoma.
The APC gene controls cell growth and division. Mutations in this gene cause cells to grow out of control. This leads to tumors. Finding APC mutations in families with FAP history is very important.
Genotype-Phenotype Correlations
FAP’s effects can differ a lot between people, even in the same family. Some APC mutations are linked to more severe FAP or a higher risk of other cancers, like hepatoblastoma.
- Attenuated FAP: Fewer polyps and cancer starts later.
- Classic FAP: Many polyps, high risk of colorectal cancer if not treated.
Screening Recommendations for FAP Families
Regular screening is key for FAP families to catch cancers early, like hepatoblastoma. Screening includes:
- Genetic testing for APC mutations in family members.
- Annual ultrasound and alpha-fetoprotein (AFP) test in kids under 5 for hepatoblastoma.
- Colonoscopies every year or so, starting in the teens, based on family history.
Early detection and management can greatly improve outcomes for FAP families.
Simpson-Golabi-Behmel Syndrome and Liver Cancer Risk
The Simpson-Golabi-Behmel Syndrome is a rare X-linked condition. It has been linked to an increased risk of tumors, including hepatoblastoma. This syndrome is marked by overgrowth before and after birth, unique facial features, and various birth defects.
Clinical Manifestations and Growth Patterns
People with Simpson-Golabi-Behmel Syndrome often have big birth weights and lengths. They also grow more during childhood.
Key Clinical Features:
- Macrosomia
- Macroglossia
- Cleft palate
- Organomegaly
- Renal and cardiac anomalies
GPC3 Gene Mutations and Hepatoblastoma Connection
The GPC3 gene, on the X chromosome, is key in Simpson-Golabi-Behmel Syndrome. Mutations or deletions in this gene are linked to the syndrome and a higher risk of hepatoblastoma.
Differential Diagnosis from Other Overgrowth Syndromes
It’s important to tell Simpson-Golabi-Behmel Syndrome apart from other overgrowth syndromes like Beckwith-Wiedemann Syndrome. This is for the right care and watch.
| Syndrome | Key Features | Genetic Basis |
| Simpson-Golabi-Behmel Syndrome | Macrosomia, macroglossia, organomegaly | GPC3 gene mutations |
| Beckwith-Wiedemann Syndrome | Macrosomia, macroglossia, omphalocele | Imprinting disorders on chromosome 11p15 |
Knowing these differences is key for giving the right care and watch for those with these syndromes.
Trisomy 18 (Edwards Syndrome) and Hepatoblastoma
Edwards Syndrome is a rare chromosomal disorder. It happens when there’s an extra copy of chromosome 18. This condition severely affects development and is linked to a rare form of liver cancer called hepatoblastoma.
Rare Association and Clinical Significance
The link between Trisomy 18 and hepatoblastoma is not common. Yet, it’s very important because both conditions are severe.
Children with Trisomy 18 face serious developmental delays and health risks. The risk of hepatoblastoma adds to their challenges.
Research shows that kids with Trisomy 18 are more likely to get tumors, including hepatoblastoma. This makes regular check-ups very important.
Proposed Mechanisms of Hepatoblastoma Development
The reasons behind hepatoblastoma in Trisomy 18 patients are complex. They involve genetics and more.
Having an extra chromosome 18 might make tumors more likely. This includes hepatoblastoma.
We need more studies to understand how this rare link works at a molecular level.
Management Considerations in Complex Medical Cases
Dealing with hepatoblastoma in Trisomy 18 patients is very hard. Their health issues are complex.
A team of experts is needed. This team should include pediatric oncologists, geneticists, and others. They work together to create a treatment plan that fits each patient’s needs.
They must think about how chemotherapy might affect these patients. They also need to plan surgeries carefully.
| Condition | Key Features | Management Considerations |
| Trisomy 18 (Edwards Syndrome) | Severe developmental delays, life-threatening medical issues | Multidisciplinary care, vigilant monitoring for tumors |
| Hepatoblastoma | Malignant liver tumor, more common in young children | Chemotherapy, surgery, liver transplantation |
| Trisomy 18 with Hepatoblastoma | Complex medical condition, increased risk of tumor | Tailored treatment plans, careful management of chemotherapy toxicity |
Li-Fraumeni Syndrome and Pediatric Liver Tumors
It’s important to know about Li-Fraumeni Syndrome and its link to liver tumors in kids. This rare genetic disorder is caused by a mutation in the TP53 gene. This mutation raises the risk of many cancers, often at a young age.
TP53 Mutations and Cancer Predisposition Spectrum
The TP53 gene helps control cell growth and stops damaged cells from growing. When this gene mutates, it loses its ability to stop cancer. This leads to a higher risk of many cancers, including soft tissue sarcomas and brain tumors.
TP53 mutations are passed down in families in an autosomal dominant way. This means just one copy of the mutated gene is enough to increase cancer risk. Genetic counseling is key for families with LFS.
Hepatoblastoma as a Component of LFS
Hepatoblastoma is a rare liver cancer mostly seen in kids. It’s part of the cancer spectrum linked to Li-Fraumeni Syndrome. For kids with a family history of LFS, regular check-ups are vital.
Comprehensive Surveillance Strategies
Because LFS increases the risk of many cancers, early detection is critical. Kids with LFS need regular ultrasound scans and AFP tests for liver cancer. The surveillance plan should match the child’s risk and family history.
Managing LFS in kids requires a team effort. Pediatric oncologists, geneticists, and other experts should work together. This team approach helps families get the support and guidance they need.
Other Rare Syndromes Associated with Hepatoblastoma
Genetic syndromes with metabolic or chromosomal issues raise the risk of hepatoblastoma. These rare conditions play a big role in the genetic risk for this cancer.
Glycogen Storage Diseases and Metabolic Predisposition
Glycogen storage diseases (GSDs) affect how the body uses glycogen. Some GSDs, like Type I, increase the risk of hepatoblastoma. Liver problems from these diseases can lead to cancer. Glycogen storage disease type I and hepatoblastoma: a case report and review of the literature. This shows the importance of watching for liver tumors in GSD patients.
| GSD Type | Enzyme Deficiency | Hepatoblastoma Risk |
| Type I | Glucose-6-phosphatase | Elevated |
| Type III | Debranching enzyme | Moderate |
| Type IV | Branching enzyme | Low |
Noonan Syndrome and RAS-MAPK Pathway Disorders
Noonan Syndrome is a genetic disorder affecting the RAS-MAPK pathway. It causes heart problems, unique faces, and a higher risk of cancers like hepatoblastoma.
The link between Noonan Syndrome and hepatoblastoma shows how important the RAS-MAPK pathway is. Mutations in this pathway can lead to cancer.
Fanconi Anemia and DNA Repair Defects
Fanconi Anemia is a rare genetic disorder with bone marrow failure and cancer risk. It’s caused by DNA repair gene mutations, leading to unstable DNA.
People with Fanconi Anemia are more likely to get cancers, including hepatoblastoma. The DNA repair problem leads to genetic damage, which can cause tumors.
Trisomy 21 (Down Syndrome) and Hepatoblastoma Risk
Trisomy 21, or Down Syndrome, increases the risk of health problems, including some cancers. While most cancers are not more common, there’s a higher risk of acute lymphoblastic leukemia. The relationship between Down Syndrome and hepatoblastoma is not well understood, with some studies suggesting a protective effect against solid tumors.
The complex relationship between Trisomy 21 and cancer risk shows the need for more research into the underlying mechanisms.
Diagnostic Approaches for Hepatoblastoma-associated Syndromes
Hepatoblastoma-associated syndromes are hard to diagnose. They need a mix of clinical skills, imaging know-how, and molecular tests. A full approach is needed, using different tools and methods.
Clinical Evaluation and Dysmorphology Assessment
Starting with a detailed medical history and physical check-up is key. Clinical geneticists are vital in spotting syndromic signs that might not be obvious. They look at growth patterns, facial features, and other signs to find syndromes linked to hepatoblastoma.
It’s also important to look at the family history. This helps find hereditary conditions that raise the risk of hepatoblastoma. This info guides further tests and treatment plans.
Imaging Studies and Biomarkers
Imaging is critical for diagnosing and managing hepatoblastoma. Abdominal ultrasound is often the first step to check liver masses. CT or MRI scans might follow to see how big the tumor is and its position. These images help plan surgery and check treatment success.
Biomarkers like alpha-fetoprotein (AFP) are also key. High AFP levels can mean hepatoblastoma, though not all cases have it. Tracking AFP levels helps see how well treatment is working and if the tumor comes back.
Genetic Testing Technologies
Genetic testing is now a big part of diagnosing these syndromes. Next-generation sequencing (NGS) lets us check many genes at once. It finds specific mutations or changes in chromosomes linked to hepatoblastoma risk.
Choosing the right genetic test depends on the suspected syndrome and symptoms. For example, testing for Beckwith-Wiedemann Syndrome might look at chromosome 11p15. Testing for APC gene mutations is key for Familial Adenomatous Polyposis (FAP).
- Next-generation sequencing (NGS) for detailed genetic analysis
- Molecular testing for specific syndromes (e.g., BWS, FAP)
- Cytogenetic analysis for chromosomal changes
By combining clinical checks, imaging, biomarkers, and genetic tests, doctors can accurately diagnose these syndromes. This helps create the right treatment plans.
Screening Protocols for High-Risk Children
Children at high risk need special screening to watch for hepatoblastoma. We suggest a detailed plan. This includes checking alpha-fetoprotein (AFP) levels and doing abdominal ultrasounds. How often and what to look for depends on the child’s risk factors and the syndrome they have.
AFP Monitoring: Frequency and Interpretation
Checking AFP levels is key in spotting hepatoblastoma. We advise AFP tests every 3-4 months until they’re 4 years old. This is because most cases happen in this age group. It’s important to understand AFP levels in the context of the child’s age, as normal values change a lot in early childhood.
Table: AFP Levels Interpretation
| Age | Normal AFP Range |
| 0-1 month | 1000-50000 ng/mL |
| 1-12 months | 10-1000 ng/mL |
| 1-4 years | <10 ng/mL |
Abdominal Ultrasound Guidelines by Syndrome
Ultrasound of the abdomen is also vital in screening for hepatoblastoma. How often ultrasounds are done can change based on the syndrome. For example, kids with Beckwith-Wiedemann Syndrome or Familial Adenomatous Polyposis might need more scans because they’re at higher risk.
Risk-Adapted Surveillance Strategies
We support using surveillance plans that match the child’s genetic syndrome and risk level. For instance, kids with certain genetic changes might need closer monitoring. On the other hand, those with lower risks might not need as many tests.
- High-risk children: AFP and ultrasound every 3 months
- Moderate-risk children: AFP and ultrasound every 6 months
- Low-risk children: Annual AFP and ultrasound
Duration of Monitoring and Age-Related Considerations
The length of monitoring should fit the child’s risk and age. Generally, we suggest keeping an eye on them until they’re at least 4 years old. This is when the risk of hepatoblastoma drops a lot. But, some high-risk kids might need to be watched longer.
By using these screening methods, we can catch hepatoblastoma early. This helps improve treatment results for high-risk children.
Treatment Considerations in Syndromic Hepatoblastoma
Treating syndromic hepatoblastoma needs a team effort. This includes surgery, chemotherapy, and sometimes liver transplantation. It’s a complex challenge that needs a custom plan for each child.
Surgical Approaches and Resectability Assessment
Removing the tumor is key in treating hepatoblastoma. Doctors check if the tumor can be removed safely. Preoperative imaging helps figure out if surgery is possible.
Chemotherapy Regimens and Toxicity Concerns
Chemotherapy is a big part of treating hepatoblastoma. It helps shrink tumors before surgery and kills any cancer left after. The right chemotherapy depends on the tumor and the child’s health.
| Chemotherapy Regimen | Toxicity Concerns |
| Platinum-based chemotherapy | Oto-toxicity, nephrotoxicity |
| Combination chemotherapy (e.g., C5VD) | Myelosuppression, risk of secondary malignancies |
Liver Transplantation Indications and Outcomes
For tumors that can’t be removed or don’t respond to treatment, liver transplant is an option. It’s a big decision based on the patient’s health and chances of recovery.
“Liver transplantation offers a potentially curative option for children with unresectable hepatoblastoma, providing a new lease on life for these patients.”
Multidisciplinary Care Coordination
Getting syndromic hepatoblastoma treated right needs a team. This team includes surgeons, oncologists, radiologists, and geneticists. Working together ensures the best care for each child.
Long-term Outcomes and Prognosis
The long-term outcomes for patients with hepatoblastoma-associated syndromes depend on many factors. These include the specific syndrome, treatment, and late effects. Improving treatment protocols is key to better patient care.
Survival Rates in Syndromic vs. Non-syndromic Cases
Survival rates for hepatoblastoma patients vary a lot. Syndromic patients face extra challenges due to their genetic conditions. This can affect treatment and prognosis.
For example, patients with Beckwith-Wiedemann Syndrome (BWS) have a higher risk of hepatoblastoma. But, early detection and tailored treatments have improved their survival rates.
“Genetic testing has changed how we manage hepatoblastoma-associated syndromes,” says a leading pediatric oncology expert. “Knowing the genetic basis helps us predict outcomes and tailor treatments.”
Late Effects and Secondary Malignancies
Late effects and secondary malignancies are big concerns for long-term survivors. Chemotherapy and radiation therapy can cause problems like cardiotoxicity and secondary cancers. Patients with Li-Fraumeni Syndrome (LFS) are at high risk of multiple primary cancers due to TP53 mutations.
- Regular surveillance for late effects is key for early detection and treatment.
- Multidisciplinary care teams are important for long-term follow-up.
- Patient education on late effects and follow-up care is vital.
Quality of Life Considerations
Quality of life is critical for long-term care of hepatoblastoma survivors. Factors include chronic health conditions, psychological well-being, and social integration. Comprehensive care plans are essential for improving quality of life.
| Aspect | Considerations |
| Physical Health | Management of late effects, chronic conditions |
| Psychological Well-being | Counseling, support groups |
| Social Integration | Education, employment support |
Transition to Adult Care
Ensuring a smooth transition to adult care is vital for hepatoblastoma survivors. This involves transferring medical records and preparing the patient for adult healthcare systems. Adult care can be complex and different from pediatric care.
Effective transition planning includes educating patients about their condition and treatment history. It also involves connecting them with adult healthcare providers who understand their needs.
Genetic Counseling for Families
Genetic counseling is key for families with hepatoblastoma-associated syndromes. It helps them grasp their risks and what genetic findings mean. We offer full support to these families.
Risk Assessment and Pedigree Analysis
Genetic counseling starts with a detailed risk assessment and pedigree analysis. We collect a family’s history to spot patterns and risks. This helps us see if these syndromes might hit other family members.
Key components of risk assessment include:
- Detailed family history
- Identification of affected family members
- Analysis of genetic testing results
Reproductive Options and Preimplantation Genetic Testing
For families with a history of these syndromes, thinking about reproductive options is vital. Preimplantation genetic testing (PGT) is a big help. It checks embryos for genetic issues before they’re implanted during IVF.
Reproductive options may include:
- Prenatal diagnosis
- Preimplantation genetic testing
- Donor gametes or embryos
Cascade Testing for Family Members
Cascade testing checks family members for a known genetic mutation. It’s key for spotting at-risk individuals early. This way, we can start early intervention or watch them closely.
| Family Member | Testing Recommendation |
| First-degree relatives | Genetic testing for known mutations |
| Second-degree relatives | Consider genetic testing based on family history |
Psychological Support and Resources
Genetic counseling also offers psychological support and resources. Dealing with the risk of these syndromes is tough. It’s important to have access to counseling and support groups.
We believe in a team effort to support families with these syndromes. Genetic counseling, medical care, and psychological support together offer the best care.
Conclusion: Advancing Care for Children with Hepatoblastoma-associated Syndromes
Genetic understanding and clinical management have greatly improved care for kids with hepatoblastoma. We’ve looked at how hepatoblastoma is linked to genetic syndromes like Beckwith-Wiedemann Syndrome and Familial Adenomatous Polyposis. Knowing these connections helps us support families better.
Managing these syndromes needs a team effort. This includes using genetic insights, watching for signs, and creating specific treatment plans. By improving care, we can make life better for kids with liver cancer and their families.
Supporting these children means working together. Pediatric oncologists, geneticists, and surgeons all play a part. As we learn more, we can make screening and treatment better. This will help these kids get the care they need.
FAQ
What is hepatoblastoma and which genetic syndromes are associated with it?
Hepatoblastoma is a rare liver tumor that mainly affects kids. It’s linked to several genetic syndromes. These include Beckwith-Wiedemann Syndrome, Familial Adenomatous Polyposis, and others.
What is the genetic basis of hepatoblastoma-associated syndromes?
The genetic basis is complex. It includes chromosomal changes, copy number variations, and mutations. Epigenetic mechanisms also play a role in its development.
How is hepatoblastoma risk stratified in patients with Beckwith-Wiedemann Syndrome?
In BWS patients, risk is assessed by clinical features and molecular subtypes. Imprinting disorders are also considered.
What is the role of genetic testing in diagnosing hepatoblastoma-associated syndromes?
Genetic testing is key in diagnosing these syndromes. It identifies specific genetic mutations or alterations.
How often should high-risk children be screened for hepatoblastoma?
Screening involves regular checks of alpha-fetoprotein (AFP) levels and ultrasound. The frequency depends on the syndrome and individual risk.
What are the treatment considerations for hepatoblastoma in children with associated genetic syndromes?
Treatment considers surgical resectability, chemotherapy, and liver transplantation. It requires a multidisciplinary approach.
What are the long-term outcomes for patients with hepatoblastoma-associated syndromes?
Outcomes depend on the syndrome, treatment, and presence of late effects or secondary malignancies.
Why is genetic counseling important for families affected by hepatoblastoma-associated syndromes?
Genetic counseling offers risk assessment, reproductive options, and cascade testing. It provides psychological support to families.
Can hepatoblastoma be associated with other rare genetic syndromes beside the well-known ones?
Yes, other rare syndromes like glycogen storage diseases and Noonan Syndrome are linked to an increased risk of hepatoblastoma.
What is the significance of AFP monitoring in screening for hepatoblastoma?
AFP monitoring is vital for early detection in high-risk children. The frequency and interpretation depend on the syndrome and individual risk.
References
- Mussa, A., & Ferrero, G. B. (2021). Screening of hepatoblastoma in Beckwith-Wiedemann syndrome: A critical review of clinical evidence. Cancers, *13*(12), 2922. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8229779/