What is amyloid protein? This essential guide explains how this protein builds up and its dangerous link to Alzheimer’s.
We face a big challenge in understanding amyloid protein. It’s a key issue in today’s medicine. Amyloidosis is a rare disease where amyloid builds up in organs, making them not work right. This happens when proteins misfold and turn into long, hard fibers.
These fibers have a special beta-sheet structure. This is what makes them different from normal proteins. Knowing where these proteins come from helps us understand their role in diseases. This includes brain diseases.
Key Takeaways
- Amyloid protein is formed through the polymerization of misfolded peptides or proteins.
- The buildup of amyloid can lead to amyloidosis, a rare disease affecting organ function.
- Understanding amyloid protein origins is key to understanding its role in diseases.
- Amyloid proteins have distinctive beta-sheet structures.
- The accumulation of amyloid proteins is linked to progressive neurodegenerative diseases.
The Science Behind Amyloid Protein

Exploring amyloid proteins sheds light on their role in health and disease. These proteins are complex and have caught the eye of medical researchers. They are linked to many diseases.
Definition and Fundamental Characteristics
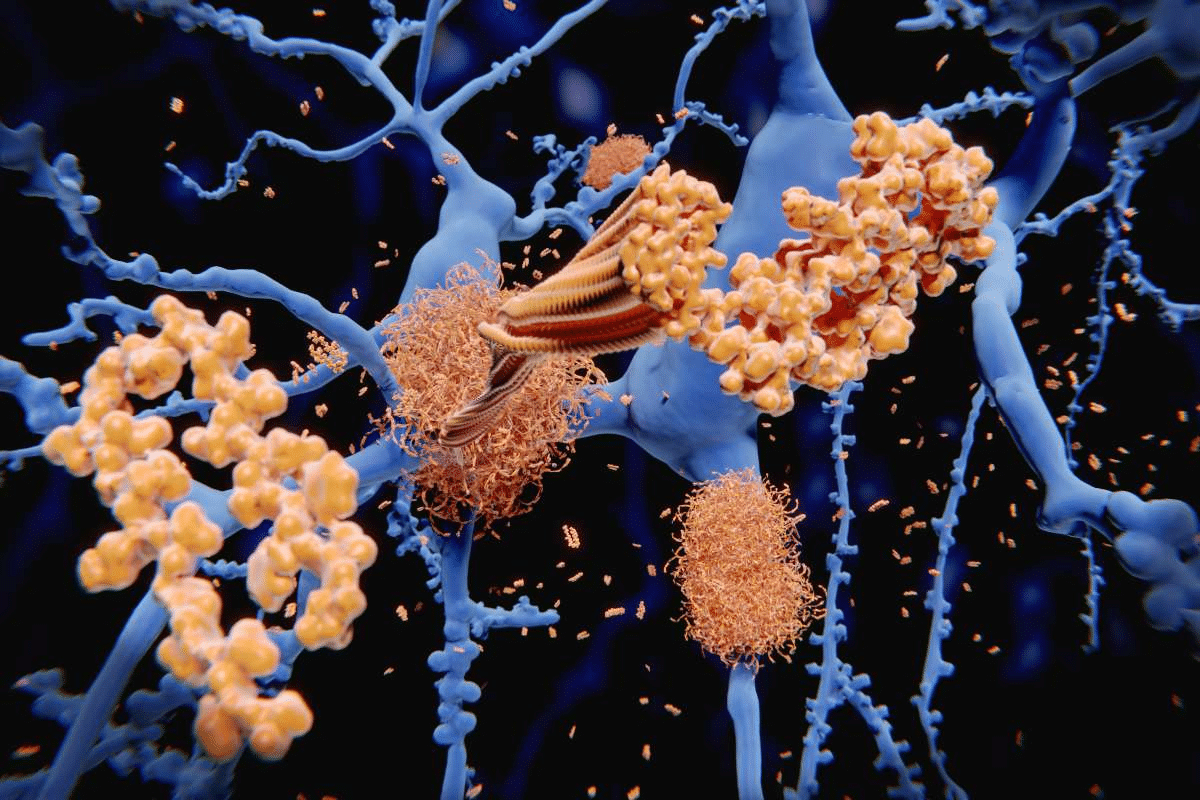
Amyloid proteins are known for their unique structure and ability to form insoluble fibrils. They are called amyloidogenic because they tend to clump into amyloid fibrils. Their key trait is forming stable, hard-to-break structures.
Amyloid beta (Aβ) peptides are a key part of amyloid plaques in Alzheimer’s disease. These peptides are 36 to 43 amino acids long. They play a big role in Alzheimer’s disease.
Distinctive Beta-Sheet Structures
Amyloid proteins are famous for their beta-sheet structures. These structures are key to their stability and insolubility. The beta-sheet shape lets amyloid proteins stack, forming long, fibrillary structures seen in amyloid deposits.
|
Characteristic |
Description |
Implication |
|---|---|---|
|
Beta-sheet structure |
Stable, insoluble conformation |
Resistance to degradation |
|
7-nanometer diameter |
Specific fibril diameter |
Characteristic of amyloid fibrils |
|
Amyloidogenic |
Propensity to form amyloid fibrils |
Association with disease pathology |
The unique beta-sheet structures and other traits of amyloid proteins are vital in disease research. They are a key focus in medical studies.
Formation Process: How Amyloid Proteins Develop
Amyloid proteins start with the misfolding of peptides or proteins. This misfolding is key because it leads to abnormal protein structures. These structures then clump together to form fibrils.
Protein Misfolding Mechanisms
Protein misfolding happens when a protein doesn’t fold right. This can be due to genetic mutations, enzymatic changes, or mistakes during protein making. These misfolded proteins can change more, leading to amyloid fibrils.
Genetic mutations can make proteins more likely to misfold. Enzymatic cleavage of proteins can also create fragments that tend to misfold and stick together.
Polymerization into Insoluble Fibers
After proteins misfold, they can turn into insoluble fibers. This happens through the stacking of beta-sheets. This step is important for making amyloid fibrils, seen in amyloid diseases.
The polymerization process starts with misfolded proteins forming oligomers. These then grow into protofibrils and mature amyloid fibrils. These fibrils are very stable and hard to break down, causing them to build up in tissues.
Knowing how amyloid protein formation works is key to finding treatments. It helps in stopping or removing these harmful proteins.
Physical Properties of Amyloid Fibrils
It’s important to know about the physical properties of amyloid fibrils. They are stable and have a unique structure. This makes them build up in tissues.
Characteristic 7-Nanometer Diameter Structure
Amyloid fibrils are about 7 nanometers wide. This size is key to identifying them. The 7-nanometer diameter helps them interact with cells in specific ways, affecting disease.
Resistance to Degradation
Amyloid fibrils are hard to break down. This is because of their stable beta-sheet structure. They resist digestion and other ways to clear them out. So, they stay in tissues for a long time, helping diseases get worse.
The table below shows the main physical properties of amyloid fibrils and how they affect disease:
|
Physical Property |
Description |
Implication for Disease |
|---|---|---|
|
7-Nanometer Diameter |
Consistent diameter of amyloid fibrils |
Facilitates specific interactions with cellular components |
|
Resistance to Degradation |
Highly stable beta-sheet structure |
Persistence in tissues, contributing to disease progression |
In summary, the physical properties of amyloid fibrils are vital. Their 7-nanometer diameter and resistance to breakdown are key. Knowing these helps us find ways to treat amyloid-related diseases.
Primary Sources of Amyloid Protein
It’s important to know where amyloid protein comes from to understand amyloid-related diseases. Amyloid proteins are involved in many diseases. They come from different biological processes.
Genetic Mutations in APP and Transthyretin
Genetic changes are key in making amyloid proteins. Mutations in APP and transthyretin genes cause amyloidosis. For example, APP gene mutations lead to abnormal amyloid-beta peptides. These are key in Alzheimer’s disease.
Transthyretin mutations cause familial amyloid polyneuropathy. This is a condition where amyloid builds up in nerves and tissues.
|
Gene |
Disease Association |
Effect of Mutation |
|---|---|---|
|
APP |
Alzheimer’s Disease |
Increased production of amyloid-beta |
|
Transthyretin |
Familial Amyloid Polyneuropathy |
Production of unstable transthyretin protein |
Enzymatic Cleavage of Precursor Proteins
Enzymatic cleavage of precursor proteins is another source of amyloid proteins. APP is cleaved by beta-secretase and gamma-secretase. This creates amyloid-beta peptides. The activity of these enzymes is key to amyloid-beta levels.
“The enzymatic processing of APP is a complex process involving multiple enzymes and regulatory pathways, highlighting the potential for therapeutic intervention.
Protein Misfolding Errors
Protein misfolding is a main cause of amyloid formation. When proteins misfold, they form insoluble fibrils. Aging, oxidative stress, and diseases can lead to misfolding.
Knowing these sources helps in making therapies. These therapies aim to reduce amyloid protein production and accumulation.
Genetic Factors Influencing Amyloid Production
Genetic factors are key to understanding amyloid production. They help diagnose and treat diseases related to amyloid. We’ll look at how genetics affects amyloid production.
Variants That Increase Production Rates
Some genetic variants make amyloid proteins produce more. For example, APP gene mutations can cause too much amyloid-beta. This is a big problem in Alzheimer’s disease. We’ll dive into how these variants boost amyloid production.
Research shows that certain mutations make amyloid precursor proteins overexpress. This increases the chance of amyloid fibrils forming.
Mutations Creating “Stickier” Protein Fragments
Genetic changes can make amyloid proteins stick together more. These sticky proteins can form insoluble fibrils easily. This is bad for disease. We’ll talk about how these changes affect amyloid proteins.
For instance, transthyretin (TTR) gene mutations can make TTR proteins more likely to cause amyloidosis. This is a hereditary condition.
Polymorphisms in Native Peptides
Genetic variations in native peptides can also affect amyloid production. These changes can make amyloid proteins more stable or prone to aggregation. We’ll see how these variations raise disease risk.
A good example is apolipoprotein E (APOE) alleles. They increase Alzheimer’s disease risk by affecting amyloid-beta aggregation.
The following table summarizes key genetic factors influencing amyloid production:
|
Genetic Factor |
Effect on Amyloid Production |
Associated Disease |
|---|---|---|
|
APP gene mutations |
Increased amyloid-beta production |
Alzheimer’s disease |
|
TTR gene mutations |
Production of “stickier” TTR proteins |
Hereditary transthyretin amyloidosis |
|
APOE polymorphisms |
Influences amyloid-beta aggregation |
Alzheimer’s disease |
In conclusion, genetics greatly influence amyloid production. They affect how fast amyloid proteins are made and how likely they are to clump together. Knowing these genetic factors is key to finding new treatments.
Environmental and Physiological Triggers
It’s important to know what triggers amyloid protein buildup to find effective treatments. Amyloidosis is a condition where amyloid proteins build up in tissues. This buildup is influenced by many factors, including the environment and our body’s functions.
Inflammatory Processes
Chronic inflammation can lead to amyloidosis. It causes more amyloid precursor proteins to be made. These proteins then turn into harmful fibrils that harm organs.
For example, conditions like rheumatoid arthritis, which cause long-term inflammation, increase the risk of amyloidosis. The inflammation can also make genes that produce amyloid more active, worsening the disease.
Oxidative Stress
Oxidative stress is another big trigger for amyloid buildup. It happens when our body can’t handle harmful compounds. These compounds can change amyloid precursor proteins, making them more likely to misfold and clump together.
Research shows that oxidative stress can help create amyloid fibrils in lab tests. Also, antioxidants can lower amyloid buildup in some models. This suggests that fighting oxidative stress could help treat amyloidosis.
Aging-Related Factors
Aging is a major risk factor for amyloidosis. As we get older, the risk of amyloid-related diseases goes up. This is because aging affects how our cells handle proteins and deal with stress.
Older cells have a harder time getting rid of misfolded proteins, including amyloid. This makes amyloid deposits build up over time.
|
Trigger |
Mechanism |
Disease Association |
|---|---|---|
|
Inflammatory Processes |
Increased production of amyloid precursor proteins |
Rheumatoid arthritis, chronic infections |
|
Oxidative Stress |
Modification of amyloid precursor proteins, making them misfold |
Alzheimer’s disease, Parkinson’s disease |
|
Aging-Related Factors |
Decline in proteostasis, increased oxidative stress |
Senile systemic amyloidosis, Alzheimer’s disease |
In conclusion, factors like inflammation, oxidative stress, and aging are key in amyloidosis. Knowing about these triggers helps us understand how to prevent and treat amyloid-related diseases.
Amyloid Proteins in Neurological Disorders
Amyloid proteins are key in many neurological diseases, affecting millions. Over 50 human diseases, like Alzheimer’s, are linked to amyloid buildup. When these proteins misfold, they form harmful clumps that disrupt cells and cause disease.
Beta-Amyloid in Alzheimer’s Disease
Beta-amyloid (Aβ) is central to Alzheimer’s disease. It forms plaques in the brain, causing damage and memory loss. Studying Aβ helps us understand and treat Alzheimer’s better.
Alpha-Synuclein in Parkinson’s Disease
Alpha-synuclein is vital in Parkinson’s disease. It clumps into Lewy bodies, which are signs of the disease. Learning about alpha-synuclein is key to finding Parkinson’s treatments.
Prion Proteins in Transmissible Spongiform Encephalopathies
Prion proteins are linked to rare, fatal brain diseases. Their misfolding creates infectious particles that spread the disease. Research on prions helps us understand protein misfolding and its effects on the brain.
Studying amyloid proteins has greatly helped us understand these diseases. More research is needed to find better treatments and improve patient care.
The Spectrum of Amyloid-Related Diseases
Amyloid proteins build up in many diseases, like systemic amyloidosis and metabolic disorders. These diseases can affect different parts of the body or just one area. Knowing about these diseases helps doctors diagnose and treat them.
Systemic Amyloidosis Types
Systemic amyloidosis happens when amyloid proteins gather in many organs. Primary amyloidosis, or AL amyloidosis, is linked to abnormal proteins in the blood. Hereditary transthyretin amyloidosis is caused by gene mutations, leading to amyloid in various organs.
These diseases can harm the heart, kidneys, liver, and nervous system. Finding the disease early is key to slowing it down and helping patients.
Localized Amyloid Conditions
Localized amyloid conditions happen when amyloid builds up in specific areas. For example, Alzheimer’s disease causes amyloid plaques in the brain. Amyloid deposits in the islets of Langerhans in the pancreas are linked to type 2 diabetes.
These conditions have unique signs and symptoms based on where the amyloid is found.
Metabolic Disorders: Type 2 Diabetes and Amylin
Type 2 diabetes involves the buildup of amylin, a hormone made by the pancreas. Amylin buildup can harm the pancreas and lead to insulin resistance.
Learning about amylin’s role in type 2 diabetes helps find new ways to treat the disease.
Pathogenic Mechanisms: How Amyloid Proteins Cause Damage
Amyloid proteins become harmful when they form prefibrillar oligomeric intermediates. These are key in causing cell damage and neurodegeneration. They are the most harmful form, leading to the worsening of amyloid-related diseases.
Prefibrillar Oligomeric Intermediates
Prefibrillar oligomeric intermediates form early in amyloid protein aggregation. They are very toxic and can harm cell membranes. Studies show they are more harmful than the mature fibrils, making them key in amyloid disease progression.
We will dive into how these intermediates harm cells and tissues. Knowing how they work is vital for finding new treatments.
Cellular Toxicity Pathways
Amyloid proteins harm cells through several ways. They can damage membranes, cause oxidative stress, and trigger inflammation. These actions can lead to cell dysfunction and death, worsening amyloid diseases.
We will look at the specific ways amyloid proteins harm cells. This will help us find new ways to treat these diseases.
Tissue Disruption and Organ Dysfunction
Amyloid proteins can disrupt organ function when they build up in tissues. In systemic amyloidosis, they can harm organs like the kidneys, heart, and liver. This can lead to organ failure. It’s important to understand how amyloid proteins harm tissues to find effective treatments.
We will discuss how amyloid proteins affect organs and tissues. This will help us understand the progression of amyloid diseases better.
Detection and Diagnostic Approaches
Finding amyloid-related diseases early is key to managing them well. New diagnostic tools have made it easier to spot these conditions.
Imaging Techniques for Amyloid Deposits
Imaging is vital for spotting amyloid in the body. PET (Positron Emission Tomography) imaging is a top choice. It uses special tracers to show where amyloid is in the body.
Pittsburgh Compound-B (PiB) is a famous tracer for brain amyloid plaques, a sign of Alzheimer’s. This tool has changed the game by helping catch diseases early and track how they progress.
Biomarkers in Blood and Cerebrospinal Fluid
Biomarkers in blood and CSF are key for diagnosing amyloid diseases. They show if amyloid proteins are present or if the body is reacting to them.
For example, high beta-amyloid 42 in CSF points to Alzheimer’s. Blood proteins can also signal systemic amyloidosis. Finding these biomarkers early can lead to better treatment and outcomes.
Genetic Testing for Amyloid-Related Disorders
Genetic tests are also vital for diagnosing amyloid diseases. Some genes make people more likely to get these conditions.
For instance, APP gene mutations cause some Alzheimer’s, while TTR gene mutations lead to hereditary amyloidosis. Spotting these genetic signs helps in early diagnosis and sometimes, prevention.
Using imaging, biomarkers, and genetics together gives a full picture of amyloid diseases. This detailed approach is key to creating effective treatment plans for each patient.
Current Research and Therapeutic Strategies
Research is changing how we see amyloid proteins and their link to diseases. As we learn more about amyloid-related disorders, new treatments are being made. These treatments aim to stop amyloid proteins and their harmful effects.
We’re getting better at understanding how amyloid proteins form and the harm they do. This knowledge is key for making good treatments.
Approaches to Prevent Amyloid Formation
Stopping amyloid formation is a big focus of research. Several methods are being looked into, including:
- Inhibiting protein misfolding: Scientists are trying to stop proteins from turning into amyloid structures.
- Modulating enzymatic cleavage: They’re working on changing enzymes to cut down on amyloid-making fragments.
- Stabilizing native protein conformations: They’re trying to keep proteins in their normal, non-amyloid forms.
These methods aim to cut down on amyloid-making proteins. This could stop amyloid deposits from forming.
Methods for Clearing Existing Deposits
Researchers are also looking into ways to remove amyloid deposits. Some promising methods include:
- Immunotherapy: Using antibodies to find and remove amyloid deposits.
- Enzyme-based degradation: Finding enzymes that can break down amyloid fibrils.
- Small molecule-based clearance: Creating small molecules to help clear amyloid deposits.
These methods could help treat patients with amyloid-related conditions.
Targeting Oligomeric Intermediates
Oligomeric intermediates are harmful forms that come from amyloid aggregation. Targeting these is a key strategy:
“The most toxic species in amyloid-related diseases are often the oligomeric intermediates, making them a prime target for therapeutic intervention.”
Scientists are searching for compounds that can target and neutralize these toxic oligomers. This could stop disease progression.
By focusing on these strategies, we’re getting closer to treating amyloid-related diseases. More research and development in this field could greatly improve patient care.
Conclusion: Future Directions in Amyloid Research
Understanding amyloid proteins is key to finding new treatments. Scientists are studying amyloid proteins to learn how they cause diseases. This research helps us understand their role in sickness.
New ways to stop amyloid from forming and remove it are being explored. Researchers aim to create treatments that target amyloid proteins directly. This could lead to better disease management.
These advancements are important for diagnosing and treating diseases. Knowing more about amyloid proteins will help us better diagnose and treat related diseases. This could greatly improve patient care.
As we learn more about amyloid proteins, we’re getting closer to new treatments. These treatments will aim to tackle amyloid proteins and the diseases they cause. This is a big step forward in medical research.
FAQ
What is amyloid protein and how is it formed?
Amyloid protein is an abnormal structure made from misfolded peptides or proteins. It forms long, insoluble fibers. This happens when proteins misfold, creating abnormal structures that can clump together.
What are the characteristic features of amyloid proteins?
Amyloid proteins have a unique beta-sheet structure. This makes them stable and hard to break down. They also have a specific 7-nanometer diameter.
What are the primary sources of amyloid protein?
Amyloid protein comes from genetic mutations, like in APP and transthyretin genes. It also comes from the cleavage of precursor proteins and protein misfolding errors. These sources lead to amyloid protein buildup in the body.
How do genetic factors influence amyloid production?
Genetic factors can increase amyloid production. This includes mutations that make proteins stickier and polymorphisms in native peptides. These factors help amyloidosis develop by promoting protein buildup.
What are the environmental and physiological triggers that contribute to amyloidosis?
Triggers like inflammation, oxidative stress, and aging can cause amyloid protein buildup. These factors lead to disease pathology.
What is the role of amyloid proteins in neurological disorders?
Amyloid proteins, like beta-amyloid and alpha-synuclein, are linked to neurological diseases. This includes Alzheimer’s, Parkinson’s, and transmissible spongiform encephalopathies.
What is the spectrum of amyloid-related diseases?
Amyloid-related diseases include systemic amyloidosis and localized amyloid conditions. Metabolic disorders like type 2 diabetes are also included. Amyloid buildup is a key feature of these diseases.
How do amyloid proteins cause damage?
Amyloid proteins damage through prefibrillar oligomeric intermediates and cellular toxicity. They also disrupt tissues. These actions contribute to disease pathology.
What are the current diagnostic approaches for amyloid-related diseases?
Diagnosing amyloid-related diseases involves imaging, biomarkers, and genetic testing. These methods are essential for managing these diseases.
What are the current therapeutic strategies for amyloid-related diseases?
Treatment strategies include preventing amyloid formation and clearing deposits. Targeting oligomeric intermediates is also key. These approaches help manage amyloid-related diseases.
What is amyloidogenic?
Amyloidogenic means a protein or peptide can form amyloid fibrils. It describes proteins that misfold and aggregate into amyloid structures.
What is the difference between amyloid and amiloyd?
“Amiloyd” is not a valid medical term. The correct term is “amyloid,” referring to abnormal protein aggregates in diseases.
References
National Center for Biotechnology Information. Amyloid Protein: Formation and Role in Amyloidosis. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3353745/