Last Updated on November 6, 2025 by
Myelodysplastic Syndrome (MDS) is a group of disorders that affect thousands of people worldwide. It is caused by poorly formed or dysfunctional blood cells. Diagnosing MDS can be hard because its symptoms are similar to other blood disorders.
Abnormal lab tests are key in diagnosing MDS. Knowing these abnormalities helps doctors identify the condition. It also helps them decide on the right treatment.
To diagnose MDS, doctors use several tests. These tests check the blood cells and bone marrow. By looking at these test results, doctors can see the specific signs of MDS in each patient.
Key Takeaways
- Abnormal lab tests are key for diagnosing Myelodysplastic Syndrome.
- MDS diagnosis involves checking blood cells and bone marrow.
- Understanding MDS diagnostic criteria is vital for accurate diagnosis.
- Diagnosing MDS needs a detailed approach.
- Lab tests help find the specific signs of MDS.
Understanding Myelodysplastic Syndrome (MDS)
Myelodysplastic syndrome (MDS) is a complex disorder that affects the bone marrow’s ability to produce healthy blood cells. This condition is characterized by the bone marrow’s failure to produce adequate, functioning blood cells, leading to various health complications.
Definition and Pathophysiology
MDS is defined by the bone marrow’s failure to produce enough blood cells, leading to peripheral blood cytopenias. The pathophysiology involves a complex interplay of genetic mutations and environmental factors that disrupt normal hematopoiesis. These genetic mutations can lead to the clonal expansion of hematopoietic stem cells, further impairing blood cell production.
The bone marrow in MDS patients often exhibits dysplastic changes, which are morphological abnormalities in blood cells that indicate a disruption in normal cell development. Understanding the pathophysiology of MDS is key to diagnosing and managing the condition effectively.
Types of MDS
MDS encompasses a heterogeneous group of disorders, classified based on specific morphological and genetic characteristics. The World Health Organization (WHO) classification system is commonly used to categorize MDS into different subtypes, including:
- MDS with single lineage dysplasia
- MDS with multilineage dysplasia
- MDS with ring sideroblasts
- MDS with isolated del(5q)
Each subtype has distinct clinical and pathological features, influencing the prognosis and treatment approach for patients.
Complete Blood Count (CBC) Abnormalities in MDS
A Complete Blood Count (CBC) is key for spotting blood cell count issues in Myelodysplastic Syndrome (MDS). It shows details about red, white blood cells, and platelets.
In MDS, CBC tests often show abnormal results. These signs point to the bone marrow problems seen in MDS.
Anemia Patterns
Anemia is common in MDS, showing up as normocytic or macrocytic. It often comes with a low reticulocyte count, showing the bone marrow isn’t making enough red blood cells.
Key features of anemia in MDS include:
- Low hemoglobin levels
- Decreased reticulocyte count
- Presence of anisocytosis and poikilocytosis on the blood smear
White Blood Cell Abnormalities
White blood cell count issues are common in MDS. Patients might have low or high white blood cell counts.
Neutropenia is a big worry, as it raises the chance of getting sick. A CBC can also spot abnormal white blood cells, like blasts.
Platelet Count Irregularities
Thrombocytopenia is another common issue in MDS patients. Platelet counts can be low, normal, or high.
Platelet count irregularities can lead to bleeding or clotting problems, depending on the level.
In summary, CBC abnormalities are vital for diagnosing and tracking MDS. Knowing about these issues helps manage the condition better.
Peripheral Blood Smear Findings
Peripheral blood smears are key in diagnosing MDS. They show dysplastic features in blood cells. This helps doctors spot the disorder’s typical morphological abnormalities.
Morphological Abnormalities in Red Blood Cells
In MDS, red blood cells show big changes. They can vary in size and shape, and sometimes have nucleated red blood cells. These signs point to MDS’s hallmark: ineffective erythropoiesis.
White Blood Cell Dysplasia
White blood cell dysplasia is a big deal in MDS. Neutrophils might look different, with hypogranularity or pseudo-Pelger-Huët anomaly. This shows the myeloid lineage is affected.
Blast Cells in Peripheral Blood
Blast cells in the blood need a closer look. They’re not just for MDS but can mean the disease is getting worse. The number of blasts helps doctors understand the disease’s stage.
Peripheral blood smears give doctors a lot of information about MDS. By looking at these smears, doctors can make important diagnoses and plans for treatment.
Bone Marrow Examination Results
Diagnosing MDS involves a detailed look at the bone marrow. This includes samples from both aspirate and biopsy. “The bone marrow examination is a cornerstone in the diagnosis of MDS, providing critical information about the disease’s pathophysiology,” as noted by hematology experts.
Aspirate Findings
The bone marrow aspirate is key for checking cell shape and size. In MDS, it often shows dysplastic changes in different cell types. These changes can affect how cells grow and mature.
Biopsy Characteristics
A bone marrow biopsy looks at the marrow’s structure and cell count. In MDS, it might show too many or too few cells. It can also find an increase in blast cells, which is important for diagnosis and predicting the disease’s course.
Cellularity and Dysplasia Assessment
Checking cell count and dysplasia is key in diagnosing MDS. Dysplasia means cells don’t develop right. How much dysplasia there is helps classify MDS types and predict outcomes.
“The presence and extent of dysplasia in the bone marrow are critical for the diagnosis and classification of MDS.”
Looking at bone marrow results is a detailed task. It needs to combine findings from both aspirate and biopsy. This is vital for a correct MDS diagnosis and understanding the disease.
MDS Diagnosis: Criteria and Classification
Understanding MDS diagnosis criteria and classification is key for managing patients well. Diagnosing MDS involves clinical evaluation, lab tests, and classification systems. These help determine the disease’s severity and prognosis.
WHO Classification System
The World Health Organization (WHO) classification system is a main tool for diagnosing and categorizing MDS. It looks at morphological features, cytogenetic abnormalities, and clinical characteristics. This helps classify MDS into different subtypes.
The WHO system has seen updates, with the latest adding new genetic and molecular insights. Now, it includes several MDS subtypes, each with its own prognosis.
Key components of the WHO classification system include:
- Morphological assessment of bone marrow and peripheral blood
- Cytogenetic analysis to identify chromosomal abnormalities
- Clinical characteristics, such as the presence of cytopenias
IPSS and IPSS-R Scoring Systems
The International Prognostic Scoring System (IPSS) and its revised version (IPSS-R) are vital for predicting MDS patient outcomes. These systems use factors like cytogenetic abnormalities, blast percentage, and cytopenias. They help forecast survival and risk of leukemia.
The IPSS-R scoring system has enhanced the original IPSS. It includes more detailed cytogenetic information and refined blast percentage and cytopenias thresholds.
Key elements of the IPSS-R scoring system include:
- Cytogenetic analysis, with categorization of abnormalities based on prognostic significance
- Assessment of bone marrow blast percentage
- Evaluation of the severity of cytopenias
Accurate MDS diagnosis and classification are vital for treatment decisions and predicting outcomes. By using the WHO classification and IPSS/IPSS-R scoring, doctors can fully understand the disease. They can then tailor management strategies to meet each patient’s needs.
Cytogenetic Abnormalities in MDS
Understanding cytogenetic abnormalities is key for diagnosing and managing Myelodysplastic Syndrome (MDS). Cytogenetic analysis shows the chromosomal changes in MDS patients. These changes affect how the disease will progress and the treatment options.

Common Chromosomal Abnormalities
Chromosomal Abnormalities in MDS:
MDS has many cytogenetic abnormalities, like deletions, translocations, and monosomies. Chromosomes 5, 7, and 8 are often involved. For example, deletions on chromosome 5’s long arm (del(5q)) are common and have specific implications for prognosis.
| Abnormality | Frequency | Prognostic Impact |
| del(5q) | Common | Favorable |
| Monosomy 7 | Frequent | Poor |
| Trisomy 8 | Common | Intermediate |
5q Deletion Syndrome
The 5q deletion syndrome is a specific type of MDS. It’s marked by a deletion on chromosome 5’s long arm. This syndrome often has a better prognosis, mainly in patients with isolated del(5q) and low blast counts. Lenalidomide has been very effective in treating this subgroup.
Prognostic Implications of Cytogenetics
Cytogenetic findings are vital for predicting MDS outcomes. The International Prognostic Scoring System (IPSS) and the revised IPSS (IPSS-R) use these findings to categorize patients by risk. Patients with high-risk cytogenetic features, like complex karyotypes or monosomy 7, face a worse prognosis.
Using cytogenetic analysis in MDS diagnosis helps not just in identifying the disease but also in predicting its course. This information is essential for making treatment decisions.
Molecular and Genetic Testing
Understanding the molecular basis of MDS is key for accurate diagnosis and treatment. Molecular and genetic testing are vital in diagnosing and managing Myelodysplastic Syndrome (MDS).
These tests have greatly improved our ability to find specific genetic mutations in MDS. This knowledge is essential for predicting outcomes and making treatment plans.
Common Gene Mutations in MDS
MDS has a complex genetic makeup, with many mutations affecting different pathways. Some common mutations include:
- Splicing factor mutations (e.g., SF3B1, SRSF2): These are linked to specific clinical features.
- DNA methylation regulators (e.g., TET2, DNMT3A): These mutations can alter gene expression and contribute to MDS.
- Transcription factors (e.g., RUNX1): Changes in these factors can disrupt blood cell production.
These mutations help in diagnosing MDS and predicting outcomes. They help categorize patients by risk level.
Next-Generation Sequencing Applications
Next-generation sequencing (NGS) has changed the MDS field. It allows for the analysis of many genes at once. NGS panels can spot a wide range of mutations, including those linked to MDS.
NGS in MDS offers several advantages:
- Comprehensive genetic profiling: NGS gives a deeper look into the genetic changes in MDS patients.
- Improved diagnostic accuracy: NGS helps differentiate MDS from other blood cancers by identifying specific mutations.
- Prognostication: The genetic data from NGS helps refine predictions and guide treatment choices.
As the field advances, molecular and genetic testing will become even more critical in managing MDS.
Flow Cytometry Analysis
Flow cytometry is key in diagnosing MDS. It helps analyze the immunophenotypic characteristics of blood cells.
This advanced lab technique looks at many cell traits in a single test. For MDS, it spots immunophenotypic abnormalities that help in diagnosis and predicting outcomes.
Immunophenotypic Abnormalities
MDS shows different immunophenotypic abnormalities. These include changes in cell surface antigens. Flow cytometry can find these changes, like:
- Abnormal expression of lineage-specific antigens
- Altered maturation patterns of myeloid and erythroid cells
- Presence of aberrant antigen expression on blast cells
Role in Distinguishing MDS from Other Conditions
Flow cytometry is vital in telling MDS apart from other blood disorders. It spots specific immunophenotypic abnormalities. This helps differentiate MDS from:
- Aplastic anemia
- Myeloproliferative neoplasms
- Acute myeloid leukemia (AML)
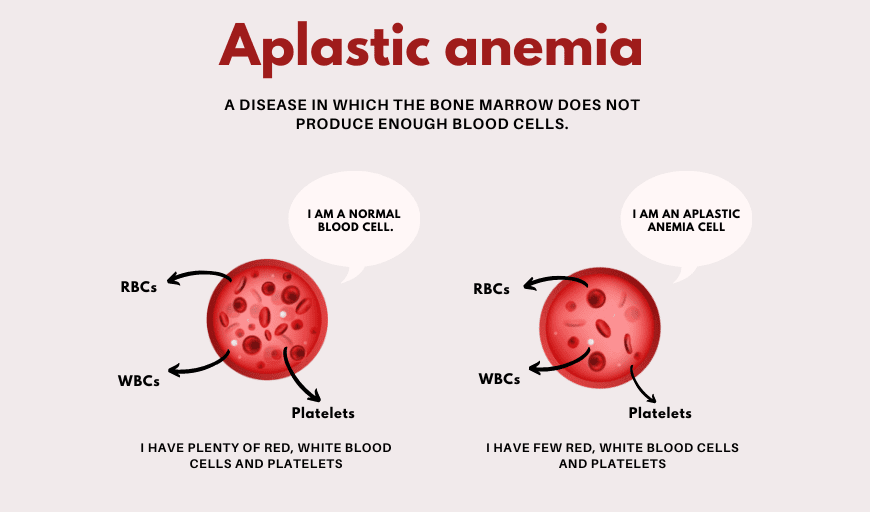
Flow cytometry’s role in MDS diagnosis shows its value in modern blood disease research. It’s a powerful tool for accurate diagnosis and managing patient care.
Biochemical Markers and Serum Abnormalities
In patients with MDS, certain biochemical markers and serum abnormalities offer valuable insights. These markers help doctors understand the disease’s severity. They also predict complications and guide treatment choices.
Serum Ferritin and Iron Studies
Serum ferritin levels are often high in MDS patients, like those with refractory anemia with ring sideroblasts (RARS). High ferritin levels suggest iron overload, often due to frequent blood transfusions. Iron studies, like serum iron and transferrin saturation, show the patient’s iron status.
Iron overload can harm organs if not managed. So, it’s key to watch serum ferritin and iron levels in MDS management.
| Parameter | Normal Range | MDS Implications |
| Serum Ferritin | 20-250 ng/mL | Elevated levels may indicate iron overload |
| Serum Iron | 60-170 mcg/dL | High levels can indicate iron overload |
| Transferrin Saturation | 20-50% | High saturation levels indicate iron overload |
Lactate Dehydrogenase (LDH) and Other Markers
Lactate dehydrogenase (LDH) is a key biochemical marker in MDS. High LDH levels suggest more cell turnover and disease activity. Though not specific to MDS, LDH helps assess disease severity when used with other tools.
Other markers, like erythropoietin levels, are also checked in MDS patients. These markers help predict how well treatments like erythropoiesis-stimulating agents will work.
Differential Diagnosis: Distinguishing MDS from Similar Conditions
It’s important to accurately diagnose MDS by distinguishing it from other bone marrow disorders. This is done through a detailed differential diagnosis. It helps to tell MDS apart from conditions that look similar.
Megaloblastic Anemia
Megaloblastic anemia is when red cells are too big and don’t work right. It’s often caused by not enough vitamin B12 or folate. It’s key to tell megaloblastic anemia apart from MDS because their treatments are different.
Here are some ways to tell them apart:
- Megaloblastic anemia has big red cells
- It doesn’t have the complex cell problems seen in MDS
- Tests for vitamin B12 and folate levels can show megaloblastic anemia
Aplastic Anemia
Aplastic anemia is when the bone marrow can’t make blood cells. MDS and aplastic anemia both have low blood counts, but they’re caused differently. MDS has problems with making cells, while aplastic anemia’s bone marrow just can’t make cells.
To tell them apart, look at:
- How full the bone marrow is and what it looks like
- If there are cell problems in MDS
- Tests like flow cytometry and cytogenetic analysis help tell them apart
Aplastic anemia usually doesn’t have the cell problems seen in MDS.
Myeloproliferative Neoplasms
Myeloproliferative neoplasms (MPNs) are diseases where too many blood cells are made. MPNs and MDS both affect blood cell production, but they’re different in many ways.
“It’s important to tell MDS apart from MPNs because of different outlooks and treatments.”
To tell MDS from MPNs, look at:
- Genetic tests for JAK2, MPL, or CALR mutations, common in MPNs
- How the bone marrow looks under a microscope
- How many blood cells are in the blood, which is often high in MPNs
In summary, to tell MDS apart from other conditions, you need to look at many things. This includes how the patient feels, lab tests, and genetic studies.
Laboratory Monitoring During MDS Treatment
MDS treatment needs close lab monitoring to check how well the treatment is working. It also helps spot if the disease is getting worse. This is key to making treatment plans better and helping patients get better.
Response Assessment Criteria
Checking how well MDS treatment is working involves looking at many lab tests. Important signs include better blood counts, needing fewer blood transfusions, and changes in the bone marrow.
Complete Blood Count (CBC) is a main test for checking treatment success. Better CBC results, like more hemoglobin, neutrophils, and platelets, show the treatment is working
.
Bone marrow examination is also very important. It shows if there are fewer blast cells and better marrow cellularity and shape.
Monitoring for Disease Progression
Lab tests are also key for catching early signs of the disease getting worse. This includes watching CBC results, bone marrow blast percentages, and genetic changes.
- Regular CBCs to monitor for worsening anemia, neutropenia, or thrombocytopenia
- Periodic bone marrow examinations to assess for increases in blast cells or development of new cytogenetic abnormalities
- Monitoring for the emergence of new cytogenetic abnormalities or molecular mutations that may indicate disease progression
Finding disease progression early can lead to better treatment and outcomes. Lab monitoring in MDS treatment is a continuous process. It needs careful analysis of test results and adjusting treatment plans as needed.
Special Considerations in MDS Laboratory Testing
Diagnosing and tracking MDS through lab tests needs a deep understanding of certain points. Getting lab results right is key for managing MDS well.
Age-Related Changes vs. MDS
One big challenge in diagnosing MDS is telling it apart from normal aging changes. As we get older, our bone marrow changes naturally. These changes can look like MDS.
Age-related changes might include mild dysplasia and small changes in blood cell counts. These can be mistaken for MDS. “Distinguishing between age-related changes and MDS requires careful evaluation of the degree and combination of abnormalities,” experts say.
“Accurate diagnosis of MDS in older adults necessitates a thorough assessment of both morphological and cytogenetic abnormalities.”
Impact of Prior Treatments on Lab Results
Prior treatments can greatly affect lab results in MDS patients. For example, chemotherapy can change blood cell counts and bone marrow cellularity. This makes it harder to understand lab results.
It’s vital to look at the patient’s treatment history when checking lab results. A leading hematologist notes, “Understanding how prior treatments affect lab results is key for accurate MDS assessment or treatment response.”
Things to think about include the type of treatment, how long it lasted, and when it happened compared to the lab tests. This helps doctors make better decisions about care and treatment plans.
By considering these special points, healthcare providers can improve MDS diagnosis and tracking. This leads to better outcomes for patients.
Conclusion
Understanding lab tests is key to diagnosing and managing Myelodysplastic Syndrome (MDS). These tests help doctors spot the unique traits of MDS. This allows them to make the right diagnosis and plan the best treatment.
Diagnosing MDS involves several lab tests. These include the Complete Blood Count (CBC), Peripheral Blood Smear, Bone Marrow Examination, and cytogenetic analysis. These tests help doctors know the type of MDS and how it will progress. This information helps them decide on the best treatment.
Getting a correct MDS diagnosis and treatment plan is all about knowing the lab tests. By using all this information, doctors can give the best care to MDS patients. This improves their life quality and treatment results.
FAQ
What is Myelodysplastic Syndrome (MDS)?
Myelodysplastic Syndrome (MDS) is a group of disorders. They are caused by poorly formed or dysfunctional blood cells. This often leads to bone marrow failure.
What are the common lab abnormalities associated with MDS?
Common lab abnormalities include anemia, neutropenia, and thrombocytopenia. Also, the presence of blast cells in the blood or bone marrow is seen.
How is MDS diagnosed?
MDS is diagnosed through several tests. These include a complete blood count (CBC), peripheral blood smear, and bone marrow aspiration and biopsy. Cytogenetic and molecular genetic testing are also used.
What is the role of the WHO classification system in MDS diagnosis?
The WHO classification system helps categorize MDS. It uses morphological, cytogenetic, and molecular features. This helps guide treatment decisions.
What is the significance of the 5q deletion syndrome in MDS?
The 5q deletion syndrome is a distinct subtype of MDS. It is characterized by a deletion on chromosome 5. It often has a favorable prognosis and responds well to certain treatments.
How does molecular and genetic testing contribute to MDS diagnosis and management?
Molecular and genetic testing identify specific gene mutations and chromosomal abnormalities. They help diagnose MDS, predict prognosis, and guide treatment decisions.
What is the purpose of flow cytometry analysis in MDS?
Flow cytometry analysis identifies immunophenotypic abnormalities in MDS. It helps distinguish MDS from other hematological disorders. It also assesses the presence of abnormal cell populations.
How are biochemical markers used in MDS?
Biochemical markers, like serum ferritin and lactate dehydrogenase (LDH), assess MDS severity. They monitor disease progression and guide treatment decisions.
What are the challenges in distinguishing MDS from other conditions?
Distinguishing MDS from other conditions is challenging. It requires a detailed diagnostic workup. Conditions like megaloblastic anemia, aplastic anemia, and myeloproliferative neoplasms can be confusing.
Why is laboratory monitoring important during MDS treatment?
Laboratory monitoring is key during MDS treatment. It assesses response to therapy and monitors for disease progression. It helps adjust treatment plans.
How do age-related changes affect MDS diagnosis?
Age-related changes can complicate MDS diagnosis. Some MDS features are seen in normal aging. A careful evaluation is needed to distinguish between age-related changes and MDS.
Can prior treatments impact MDS lab results?
Yes, prior treatments can affect MDS lab results. It’s important to consider the effects of previous therapies when interpreting lab findings.
What is the prognosis for patients with MDS?
The prognosis for MDS patients varies widely. It depends on the subtype, cytogenetic abnormalities, and other factors. Some patients have a relatively indolent course, while others may progress to aggressive disease.