We are seeing big steps forward in treating T cell malignancies, a tough group of blood cancers. CAR T cell therapy has become a game-changer, mainly for hard-to-treat cases.
Looking ahead to 2025, the FDA is making changes to CAR T cell therapy rules. These updates aim to make treatment better and more accessible for patients. Key changes include dropping REMS for certain CAR T cell treatments and working on CAR T cells that target more than one cancer type.
At Liv Hospital, we’re all about top-notch healthcare that puts patients first. Our team keeps up with the newest in CAR T cell therapy. This way, we can give our patients the best care possible.
Key Takeaways
- FDA updates have improved patient access to CAR T cell therapies.
- Elimination of REMS for autologous CAR T cell treatments enhances treatment safety.
- Multi-targeted CAR T cells are being developed to tackle various T cell malignancies.
- CAR T cell therapy has shown transformative results in refractory cases.
- Liv Hospital is at the forefront of adopting these advancements for patient care.
The Current Landscape of T Cell Malignancies
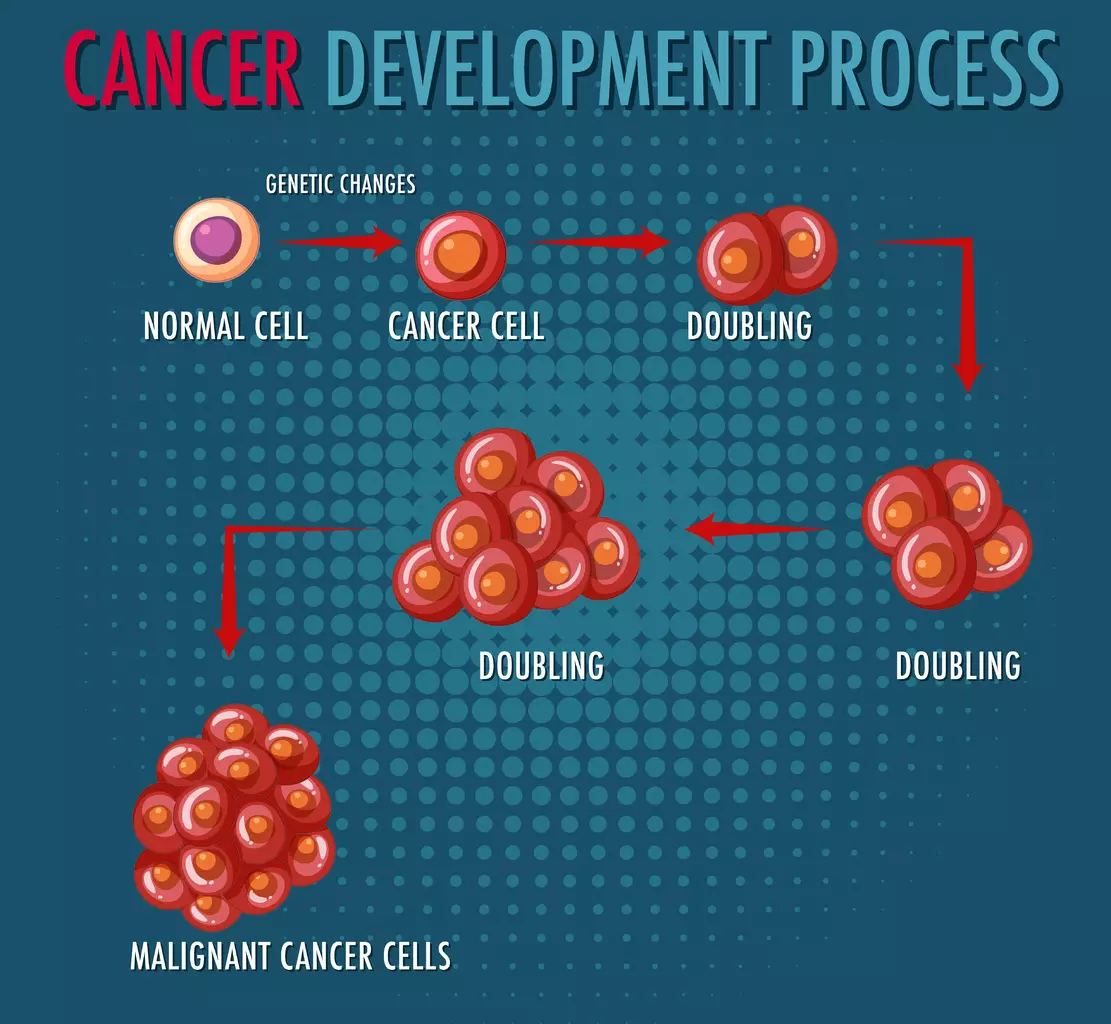
T cell malignancies are complex disorders that affect the immune system. They happen when T cells, key to our immune response, grow out of control.
Common Types and Classification
These malignancies include diseases like peripheral T-cell lymphoma (PTCL), mycosis fungoides, and T-cell acute lymphoblastic leukemia (T-ALL). Each type has its own traits and treatment challenges. For example, PTCL is a group of aggressive lymphomas with a tough prognosis.
Doctors classify T cell malignancies by the T cell type and its maturity stage. This helps in understanding the disease and planning treatment.
Challenges in Traditional Treatment Approaches
Traditional treatments like chemotherapy and radiation have their limits. They can cause serious side effects and may not work well. T cell malignancies often resist these treatments, leading to relapse and poor results.
We need new, targeted therapies to help patients. New treatments, like CAR T cell therapy, show promise in overcoming these challenges.
Understanding CAR T Cell Therapy for T Cell Malignancies
CAR T cell therapy is a new hope for patients with T cell malignancies. It changes a patient’s T cells to attack cancer cells. This makes treatment more personal and effective.
Mechanism of Action
The way CAR T cell therapy works is both complex and interesting. It starts with taking T cells from the patient’s blood or bone marrow. These T cells are then changed to find and destroy cancer cells.
After being changed, the T cells grow and attack cancer cells. This can greatly reduce or even remove tumors.
The steps include:
- T cell extraction and isolation
- Genetic modification to introduce the CAR
- Expansion of CAR T cells
- Reinfusion of CAR T cells into the patient
Historical FDA Approvals
The FDA has approved CAR T cell therapies for several blood cancers. The first was tisagenlecleucel (Kymriah) in 2017 for B cell lymphomas. More approvals have followed, covering more types of blood cancers.
These approvals come from strong clinical trials. The FDA keeps checking these treatments for safety and effectiveness. They give updates to doctors and patients.
As CAR T cell therapy grows, we’ll see more improvements. It might help more patients with T cell lymphoma and leukemia.
FDA Update #1: Elimination of Risk Evaluation and Mitigation Strategies (REMS)
The FDA has made a big change by removing REMS for autologous CAR T cell therapies. This move shows more trust in these treatments for T cell cancers. We’ll look at what REMS used to be and why it’s no longer needed.
Previous REMS Requirements for Autologous CAR T Therapies
Before, CAR T cell therapies had strict REMS rules to lower risks. These rules included mandatory training for healthcare providers, patient monitoring protocols, and strict distribution controls. The goal was to make sure CAR T cell therapies were safe, despite risks like cytokine release syndrome (CRS) and neurological toxicities.
Clinical Experience Supporting Safety Protocol Changes
The FDA decided to drop REMS after seeing lots of clinical data. Real-world evidence and clinical trial data showed managing risks was possible. This led the FDA to believe REMS was no longer needed.
Removing REMS makes it easier for patients with T cell cancers to get treated. It also lets doctors focus more on patient care, not just following rules.
This change by the FDA makes CAR T cell therapies more accessible. It’s a big step towards helping more patients get these life-saving treatments.
FDA Update #2: Expanded Patient Access Through Streamlined Monitoring
The FDA has made CAR T cell therapy more accessible. This is a big win for patients with T cell malignancies. Before, the treatment’s complexity limited its use.
Historical Access Barriers
Getting CAR T cell therapy was hard due to complex rules and strict monitoring. Only about 2 in 10 eligible patients got it. This was because it was mainly available in specialized centers.
Key barriers included:
- Complex patient selection criteria
- Stringent monitoring requirements
- Limited availability of treatment centers
This gap meant many patients missed out on a potentially life-saving treatment.
New Monitoring Requirements
The FDA has made it easier to monitor CAR T cell therapy. This change has opened up more places for treatment, including community centers.
| Monitoring Aspect | Previous Requirements | New Requirements |
|---|---|---|
| Monitoring Frequency | Daily monitoring for 7-10 days post-treatment | Monitoring every other day for 7-10 days |
| Location Requirements | Patients required to stay within 30 minutes of treatment center | Flexibility to travel, with remote monitoring options |
| Reporting Requirements | Manual reporting of patient data | Automated reporting through digital platforms |
Now, more patients can get CAR T cell therapy, even in community settings. This change is expected to lead to better patient outcomes and more use of this therapy.
As we keep watching, it’s clear that making monitoring easier has been key in getting more patients access to this new treatment.
FDA Update #3: Development of Multi-Targeted CAR T Cells
Multi-targeted CAR T cells are a big step forward in CAR T cell therapy, the FDA says. They are key to tackling the tough challenges of T cell cancers. This is because they help fight back against cancer’s defenses.
Addressing Tumor Resistance Mechanisms
Tumor resistance is a big problem for CAR T cell therapy. Single-target CAR T cells can fail as cancer cells change and lose the target. But, multi-targeted CAR T cells aim to solve this by hitting multiple targets at once. This makes it harder for cancer to hide and resist treatment.
This new way of treating T cell cancers is changing the game. By targeting more than one antigen, these treatments could lead to better results and a better outlook for patients. This is great news for those with T cell cancers that don’t respond well to other treatments.
Current Clinical Trials
Many clinical trials are underway to test multi-targeted CAR T cells in T cell cancer patients. These studies are looking at how well these treatments work and how safe they are. The early signs are encouraging, showing good results and manageable side effects.
As research keeps moving forward, multi-targeted CAR T cells will play a bigger role in treating T cell cancers. The FDA’s work in reviewing and approving these treatments is key to getting them to patients.
FDA Update #4: Revised Post-Treatment Lifestyle Restrictions
The FDA has updated the rules for life after CAR T cell therapy. This change shows a better understanding of how the treatment affects patients. The goal is to keep patients safe while also improving their quality of life during recovery.
New Driving Guidelines
The FDA has new rules for driving after CAR T cell therapy. Before, patients couldn’t drive for weeks because of the risk of neurological side effects. Now, they can drive sooner if they’re not too tired or confused.
Key aspects of the new driving guidelines include:
- Patients should be symptom-free for at least 7 days before resuming driving.
- Patients with a history of neurological issues may need to wait longer before driving again.
- It’s recommended that patients have a follow-up assessment with their healthcare provider before driving.
Changes to Proximity Restrictions
The FDA has also updated the rules for being close to others after CAR T cell therapy. Now, patients can be closer to others because the risk of passing on the treatment or causing harm is lower.
| Restriction Type | Previous Guidelines | Revised Guidelines |
|---|---|---|
| Proximity to Immunocompromised Individuals | Avoid for 6 months | Avoid for 3 months |
| Public Places | Avoid crowded areas for 6 weeks | No specific restrictions |
| Travel Restrictions | Avoid travel for 2 months | Assess on a case-by-case basis |
These updates show a better understanding of CAR T cell therapy’s effects. By relaxing some rules, the FDA wants to make life better for patients. They focus on safety while improving quality of life.
FDA Update #5: Implementation of Boxed Warnings for Key Risks
The FDA has added boxed warnings for CAR T cell therapy. This is a big step to make sure patients are safe. These warnings point out the main risks of this new treatment.
The FDA is serious about keeping patients safe. They’ve put boxed warnings on CAR T cell therapy. This is to make sure everyone knows about the dangers.
Two big risks are cytokine release syndrome (CRS) and neurological toxicity. These are serious problems that can happen with this treatment.
Cytokine Release Syndrome (CRS) Warnings
CRS is a serious condition that can happen after getting CAR T cell therapy. The FDA warns about the dangers of CRS. It can cause:
- High fever
- Fatigue
- Nausea
- Hypotension
Doctors need to watch for CRS signs closely. They should have a plan ready to handle it.
Neurological Toxicity Warnings
Neurological toxicity is another big risk with CAR T cell therapy. The FDA warns about severe brain problems. These can include:
- Encephalopathy
- Seizures
- Cerebral edema
Patients and their families need to know the signs of brain problems. They should get help right away if they see these signs.
Adding these warnings is a big step to make CAR T cell therapy safer. It helps doctors and patients know the risks. This way, we can help people with T cell cancers get better.
FDA Updates #6 and #7: Reimbursement Changes and Manufacturing Improvements
The FDA has made two big moves to help CAR T cell therapy. They’ve created new ways to pay for treatments and made making them easier. These changes aim to help more patients with T cell cancers get the treatment they need.
Update #6: New Reimbursement Pathways
The FDA has teamed up with healthcare and insurance to make CAR T cell therapy cheaper. They’ve set up new reimbursement pathways to help patients. These paths make it easier to get the financial help needed.
One big change is that more patients with T cell leukemia will get covered. This means more people can get the treatment they need.
Update #7: Streamlined Manufacturing Processes
The FDA has also made CAR T cell therapy production faster. They’ve introduced streamlined manufacturing processes to speed up production. This means patients will get their treatments sooner.
They’re using advanced manufacturing technologies and better production methods. This will cut down the wait time for patients, which is very important for treating T cell cancers quickly.
These changes are a big step forward for treating T cell leukemia and other cancers. The FDA is making CAR T cell therapy more available and effective for those who need it.
Conclusion: The Transforming Landscape of T Cell Malignancy Treatment
We’ve seen big changes in treating T cell malignancies thanks to new FDA updates on CAR T cell therapy. These updates have made treatments better and more available to patients.
Removing Risk Evaluation and Mitigation Strategies (REMS) and making monitoring easier has helped more people get CAR T cell therapy. Also, new CAR T cells that target multiple areas have helped fight tumor resistance. This has made treatments even more effective.
As we keep improving CAR T cell therapies, the future for treating T cell malignancies is bright. We’re dedicated to providing top-notch healthcare and support to patients worldwide. This ensures they get the best care for their T cell malignancies.
What are T cell malignancies?
T cell malignancies are cancers that affect T cells, a key part of our immune system. They include diseases like peripheral T-cell lymphoma and mycosis fungoides. These are types of leukemia and lymphoma.
How does CAR T cell therapy work?
CAR T cell therapy is a new way to fight cancer. It changes a patient’s T cells to find and attack cancer cells. Then, these changed T cells are given back to the patient to fight the cancer.
What is the significance of the FDA’s elimination of REMS for autologous CAR T therapies?
Removing REMS makes CAR T cell therapy easier to use. It helps more people get this treatment by making safety checks simpler.
How have the FDA’s updates improved patient access to CAR T cell therapy?
The FDA’s changes have made it simpler for patients to get CAR T cell therapy. They also made it easier for patients to recover from treatment.
What are multi-targeted CAR T cells, and how do they work?
Multi-targeted CAR T cells can find and attack many cancer cells at once. This makes treatment more effective and reduces the chance of cancer cells becoming resistant.
What are the key risks associated with CAR T cell therapy, and how are they managed?
CAR T cell therapy can cause problems like cytokine release syndrome and neurological issues. The FDA has added warnings to help patients and doctors understand these risks.
How have reimbursement changes and manufacturing improvements impacted CAR T cell therapy?
The FDA’s updates have made CAR T cell therapy more affordable and easier to make. This has made it more accessible and efficient for patients.
What are the most common types of T cell malignancies?
The most common T cell malignancies are peripheral T-cell lymphoma and mycosis fungoides. There are also other types of T-cell leukemia and lymphoma.
What are the symptoms of T cell malignancies?
Symptoms can vary but often include swollen lymph nodes, fever, weight loss, and skin lesions. They depend on the type and stage of the disease.
How are T cell malignancies typically treated?
Treatment usually involves chemotherapy, radiation, and immunotherapy. CAR T cell therapy is a new, promising option for some T cell malignancies.
What is the prognosis for patients with T cell malignancies?
The outlook varies based on the disease type, stage, and treatment success. CAR T cell therapy has shown to improve outcomes for some T cell malignancies.