Last Updated on October 21, 2025 by mcelik
At Liv Hospital, we lead in cancer treatment, using the newest in immunotherapy. CAR T-cell therapies are making waves because they might wipe out some cancers for good. This is a big deal for those fighting cancer.
We’re using chimeric antigen receptor technology to make the immune system attack cancer cells directly. This is a major step forward in treating cancer, giving hope to those with few options.
We’re diving into CAR T cells, looking at the facts from the latest studies. Our aim is to share a full picture of this new treatment and its role in fighting cancer.
Key Takeaways
- CAR T-cell therapies offer a promising approach to cancer treatment.
- This innovative therapy leverages the immune system’s natural ability to fight cancer.
- Liv Hospital is at the forefront of CAR T-cell therapy, delivering compassionate, patient-centric care.
- CAR T cells are engineered to specificially target and destroy cancer cells.
- The latest research and clinical findings support the promise of CAR T-cell therapy.
The Revolutionary Approach to Cancer Treatment

CAR T-cell therapy has changed cancer treatment by making it more personal. It uses a patient’s own T cells to fight cancer. This makes treatment fit each person’s needs.
Paradigm Shift in Immunotherapy
CAR T-cell technology is a big step forward in fighting cancer. It makes T cells better at finding and killing cancer cells. This has helped many people with blood cancers who didn’t get better with other treatments.
The FDA approved CAR T-cell therapies in 2017. This was a big moment for cancer treatment. It showed how CAR T-cell technology could change immunotherapy for the better.
Key benefits of CAR T-cell therapy include:
- Personalized treatment: Tailored to the individual patient’s immune cells.
- Targeted approach: Designed to attack cancer cells directly.
- Potential for durable responses: Could lead to long-term remission.
Personalized Medicine at the Cellular Level
CAR T-cell therapy is all about personalized medicine. It uses a patient’s own T cells for treatment. This makes the treatment more likely to work well.
The process starts with collecting and isolating T cells. Then, they are genetically modified and expanded. After that, they are given back to the patient. This complex process needs a lot of skill and care.
The future of CAR T-cell therapy looks bright. Researchers are working to make it even better. They want to use it for more cancers and solve the challenges it faces.
Key Fact 1: What Chimeric Antigen Receptor CAR T Cells Actually Are
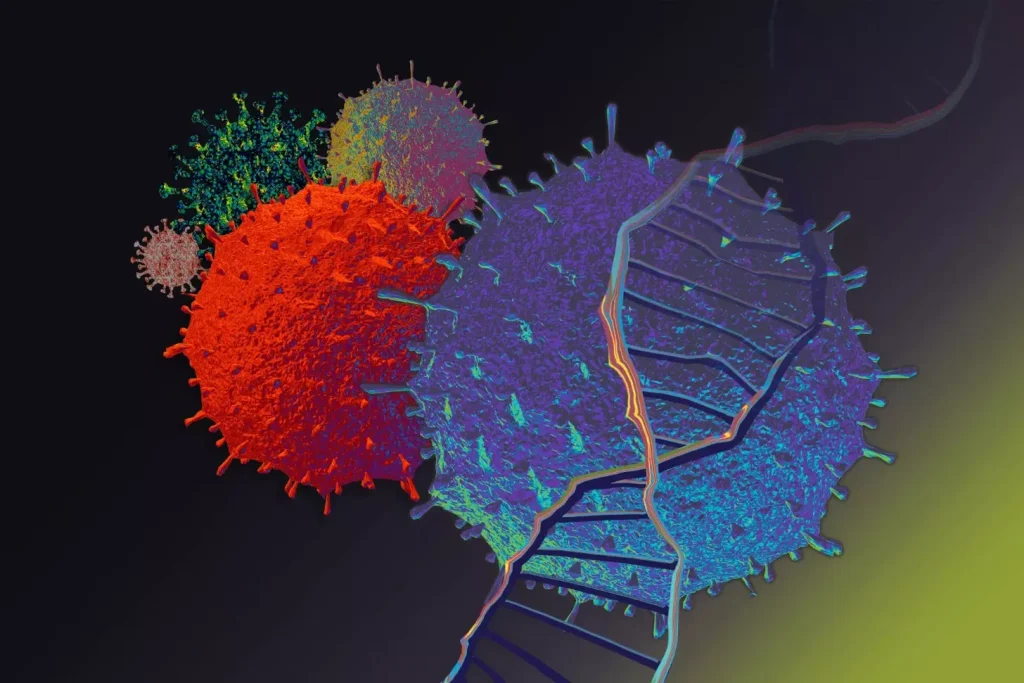
CAR T cell therapy changes a patient’s T cells to fight cancer better. It’s a new way to treat cancer, working well for some leukemia and lymphoma patients.
Definition and Basic Concept
CAR T cells are T cells that have been changed to find and kill cancer cells. T cells are the body’s main fighters against infected and diseased cells. This therapy uses T cells to target cancer cells.
First, T cells are taken from the patient. Then, they are changed to make a CAR that finds cancer cells. After that, these modified T cells are put back into the patient.
The CAR finds a specific antigen on cancer cells. When CAR T cells find these cells, they attack and kill them. This method is precise, aiming at cancer cells while protecting healthy ones.
How They Differ from Natural T Cells
Natural T cells are key in fighting off infections and diseases. But, cancer cells can hide from them. CAR T cells are different because they are made to find and kill cancer cells.
- CAR T cells are more targeted: They are made to find specific antigens on cancer cells, protecting healthy cells.
- CAR T cells are more potent: Their genetic change makes them better at growing and staying in the body, giving a strong fight against cancer.
- CAR T cells can overcome some evasion mechanisms: They can find and kill cancer cells, even when they try to hide from the immune system.
In short, CAR T cells are a big step forward in cancer treatment. They use the immune system in a focused and powerful way. Their ability to find and destroy cancer cells makes them a key treatment for some cancers.
Key Fact 2: The Engineering Process Behind CAR T Cell Therapy
At the core of CAR T-cell therapy is a detailed engineering process. It makes personalized cancer treatments possible. This process changes a patient’s T cells into strong fighters against cancer.
Collection and Isolation of Patient T Cells
The first step is collecting a patient’s T cells. This is done through leukapheresis. It draws blood, separates white blood cells, and keeps the T cells. These T cells then go to a lab for more work.
In the lab, the T cells are cleaned and sorted. This step is key. It makes sure mostly T cells are modified, reducing risks.
Genetic Modification Techniques
Next, the T cells get a genetic makeover. They get a chimeric antigen receptor (CAR) gene. This gene lets them find and attack cancer cells.
This genetic change is precise. It goes through many checks to make sure it works right and the cells are clean.
Expansion and Reinfusion Process
After the genetic change, the T cells grow in number. This takes days to weeks. They grow in a special place that helps them multiply.
When there are enough, the T cells are mixed into a product. The patient might get some chemotherapy first. This clears the way for the T cells to work better.
Putting the T cells back into the patient is easy. It’s like a blood transfusion. The T cells then find and attack cancer cells in the body.
| Step | Description | Key Considerations |
|---|---|---|
| Collection and Isolation | T cells are collected from the patient through leukapheresis and then isolated and purified. | Ensuring high purity of T cells is critical. |
| Genetic Modification | T cells are genetically modified to express CAR using viral vectors. | Quality control checks are essential to ensure correct CAR expression. |
| Expansion and Reinfusion | CAR T cells are expanded in number and then reinfused into the patient. | Conditioning chemotherapy may be used to enhance CAR T cell expansion and function. |
For more detailed information on CAR T-cell therapy, you can visit the National Cancer Institute’s page on CAR T. It offers deep insights into CAR T-cell research and treatment.
Key Fact 3: How CAR T Cells Target and Destroy Cancer
CAR T cell therapy is a new way to fight cancer. It changes T cells to find and attack cancer cells better.
Antigen Recognition Mechanism
CAR T cells are made to find specific antigens on cancer cells. They use a special receptor called CAR to do this. This helps them avoid harming healthy cells.
The CAR has parts that work together when it finds an antigen. This starts a chain of events that kills the cancer cell.
Activation of Immune Response
When CAR T cells find their target, they get activated. This makes more CAR T cells and releases cytokines. This is key for a strong fight against cancer.
| Component | Function |
|---|---|
| CAR | Antigen recognition and binding |
| T cell activation | Proliferation and cytokine release |
| Cytokines | Enhance immune response |
Persistent Anti-Cancer Memory
CAR T cell therapy gives the immune system a lasting memory against cancer. This means it can protect against cancer coming back. This is why the treatment’s effects can last a long time.
We are in a new era of cancer treatment with CAR T cell therapy. It brings hope to patients who had few options before.
Key Fact 4: FDA-Approved CAR T Cell Therapies
We are seeing a big leap in cancer treatment with the FDA’s approval of CAR T-cell therapies. This breakthrough has opened new doors for patients with certain blood cancers. It shows how fast immunotherapy is advancing.
Timeline of Breakthrough Approvals
The path to FDA approval for CAR T-cell therapies has been amazing. The first one was approved in 2017, a big step. Many more have followed, giving patients more options.
- 2017: Tisagenlecleucel (Kymriah) was approved for relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL) in patients up to 25 years old.
- 2018: Axicabtagene ciloleucel (Yescarta) received approval for relapsed or refractory large B-cell lymphoma.
- 2020: Lisocabtagene maraleucel (Breyanzi) was approved for relapsed or refractory large B-cell lymphoma.
- 2021: Idecabtagene vicleucel (Abecma) received approval for relapsed or refractory multiple myeloma.
Currently Available Commercial Products
Several CAR T-cell therapies are now on the market. Each has its own features and approved uses. The main ones available are:
| Therapy Name | Brand Name | Manufacturer |
|---|---|---|
| Tisagenlecleucel | Kymriah | Novartis |
| Axicabtagene ciloleucel | Yescarta | Kite Pharma (Gilead) |
| Lisocabtagene maraleucel | Breyanzi | Bristol Myers Squibb |
| Idecabtagene vicleucel | Abecma | Bristol Myers Squibb |
Approved Indications and Usage
Each FDA-approved CAR T-cell therapy has specific uses. It’s important for doctors and patients to know these to make good choices.
The approved uses include:
- Relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL) in patients up to 25 years old (Tisagenlecleucel).
- Relapsed or refractory large B-cell lymphoma (Axicabtagene ciloleucel and Lisocabtagene maraleucel).
- Relapsed or refractory multiple myeloma (Idecabtagene vicleucel).
These therapies have shown great results in trials, giving hope to those with few options. As research goes on, we’ll see more progress in CAR T-cell therapy. It might help more types of cancer in the future.
Key Fact 5: Remarkable Success Rates in Blood Cancers
CAR T-cell therapy has changed how we treat blood cancers. It brings new hope to patients. This therapy is leading the way in immunotherapy.
It has made a big difference in treating leukemia, lymphoma, and multiple myeloma. Let’s look at these achievements.
Leukemia Remission Statistics
CAR T-cell therapy is very effective in treating leukemia. Studies show it works well for patients with relapsed or refractory acute lymphoblastic leukemia (ALL). For example, tisagenlecleucel has a complete remission rate of up to 90% in young patients with B-cell ALL.
This shows CAR T-cell therapy’s power in changing leukemia treatment. It not only boosts remission rates but also keeps patients in remission for a long time.
Lymphoma Treatment Outcomes
CAR T-cell therapy has also improved lymphoma treatment. Axicabtagene ciloleucel and tisagenlecleucel are approved for certain lymphomas.
- Studies show these therapies work well, with response rates from 50% to over 80% in relapsed or refractory large B-cell lymphoma.
- Some patients stay in remission for a long time.
- These therapies target and kill lymphoma cells, giving hope to those with few options.
Multiple Myeloma Response Data
Multiple myeloma, a blood cancer, is also being treated with CAR T-cell therapy. Early trials show promising results, with therapies targeting BCMA showing great activity in heavily treated patients.
- Response rates are as high as 80-100% in some studies.
- Deep responses, including stringent complete responses, are encouraging.
- But, we need to keep studying to understand response durability and relapse risk.
In summary, CAR T-cell therapy has shown great success in treating blood cancers. This includes leukemia, lymphoma, and multiple myeloma. As research grows, we expect even better treatment outcomes and care for patients.
Key Fact 6: Understanding CAR T Cell Therapy Side Effects
CAR T-cell therapy brings hope to cancer patients. But, it’s important to know the side effects. Managing these side effects is key to good care.
Cytokine Release Syndrome
Cytokine release syndrome (CRS) is a major side effect. It happens when T cells release a lot of cytokines. This causes a big inflammatory response in the body.
Symptoms can be mild or very serious, like fever, low blood pressure, and organ problems.
We treat CRS with supportive care and, for severe cases, anti-cytokine therapies like tocilizumab. Quick action is vital to avoid serious issues.
Neurological Toxicities
Neurological toxicities, or ICANS, are another big worry. Symptoms include confusion, tremors, and in severe cases, seizures or brain swelling. The exact cause is being studied, but it’s thought to be due to cytokine release and inflammation.
Managing these toxicities means watching closely and providing supportive care. Corticosteroids might be used to reduce inflammation. We also try to lessen these effects with specific medicines and adjusting the CAR T-cell dose.
Management Strategies for Adverse Events
Handling CAR T-cell therapy side effects needs a team effort. Early detection and action are key. Creating personalized treatment plans is essential, based on the patient’s risk and how they react to therapy.
Also, research into new ways to reduce side effects is ongoing. This includes making new CAR T-cell designs that are safer and using treatments to prevent severe side effects.
Key Fact 7: The Cost and Accessibility Landscape
The cost of CAR T-cell therapy is a big worry for patients and healthcare systems around the world. As this treatment gets better, knowing its cost and how to get it is key for everyone involved.
Price Range for Approved Therapies
CAR T-cell therapies are very pricey. The cost for approved treatments can vary a lot.
| Therapy Name | Manufacturer | Approved Indication | Price (USD) |
|---|---|---|---|
| Tisagenlecleucel | Novartis | Relapsed/Refractory B-cell ALL | $373,000 |
| Axicabtagene ciloleucel | Gilead | Relapsed/Refractory DLBCL | $373,000 |
Insurance Coverage Considerations
Insurance for CAR T-cell therapy varies a lot. Most big insurance companies cover it, but how much they cover can differ.
“The cost of CAR T-cell therapy is a significant barrier to access, but insurance coverage can mitigate this burden.” – Dr. [Last Name], Hematologist/Oncologist
Global Availability Challenges
Getting CAR T-cell therapies worldwide is hard. It’s because of making them, getting approval, and the state of healthcare.
In conclusion, the cost and getting CAR T-cell therapy are big challenges. Understanding these issues is key to making this life-saving treatment more accessible to patients.
Key Fact 8: Challenges in Treating Solid Tumors
Using CAR T-cell therapy on solid tumors is tough due to several reasons. These include picking the right antigen and getting past the tumor’s defenses. CAR T-cell therapy works well for some blood cancers but faces big hurdles in solid tumors.
Tumor Microenvironment Barriers
The tumor microenvironment (TME) makes CAR T-cell therapy hard in solid tumors. The TME can block CAR T cells from finding and killing cancer cells. Immunosuppressive cells like regulatory T cells and myeloid-derived suppressor cells weaken CAR T cells.
Also, solid tumors’ structure makes it hard for CAR T cells to get in. The dense extracellular matrix and abnormal vasculature in tumors block CAR T cells from reaching cancer cells.
Antigen Selection Difficulties
Finding the right antigen is key for CAR T-cell therapy. In solid tumors, picking a tumor-specific antigen is hard. Antigen heterogeneity and loss let cancer cells avoid CAR T-cell attack.
Current Research Approaches
Scientists are working on new ways to beat CAR T-cell therapy challenges in solid tumors. They’re looking into:
- Creating next-generation CAR T cells that last longer and fight better.
- Using CAR T-cell therapy with other treatments, like checkpoint inhibitors, to fight the TME.
- Improving antigen selection with advanced analysis.
- Helping CAR T cells get into solid tumors better with new delivery methods.
By tackling these issues, we might be able to help more cancers, including solid tumors, with CAR T-cell therapy.
Key Fact 9: The Future Evolution of CAR T Technology
Next-generation CAR T cells are being developed to overcome current limitations and improve patient outcomes. The field of CAR T-cell therapy is rapidly advancing. Several innovative approaches are being explored to enhance its efficacy and accessibility.
Next-Generation CAR Designs
Researchers are working on improving CAR T-cell designs. They aim to enhance their ability to recognize and target cancer cells more effectively. Some of the advancements include:
- Armored CARs: These are designed to overcome the immunosuppressive tumor microenvironment.
- Dual-Targeting CARs: These can target multiple antigens, reducing the likelihood of tumor escape.
- Switchable CARs: These allow for control over CAR T-cell activity through the administration of switch molecules.
These next-generation CAR designs aim to improve the efficacy and safety of CAR T-cell therapy.
Off-the-Shelf CAR T Products
The development of off-the-shelf CAR T products is another significant area of research. These products can be manufactured in advance. They can be made available for use without the need for patient-specific production, potentially reducing costs and increasing accessibility.
Some benefits of off-the-shelf CAR T products include:
- Faster availability for patients
- Reduced manufacturing costs
- Increased accessibility for a broader patient population
Expanding Treatment Indications
CAR T-cell therapy is being explored for a wider range of indications beyond its current approvals for certain hematological malignancies. Research is ongoing to assess its potential in treating solid tumors and other types of cancers.
The expansion of treatment indications is expected to provide new therapeutic options for patients with various types of cancer.
Patient Journey Through CAR T Cell Therapy
Understanding the journey through CAR T-cell therapy is key to better treatment results. This path includes important stages from the first check-up to aftercare.
Eligibility and Selection Process
The first step is figuring out if a patient can get CAR T-cell therapy. Eligibility criteria look at the cancer type, how far it has spread, past treatments, and overall health. We check these carefully to make sure the therapy will help.
Patients get a detailed check-up. This includes looking at their medical history, doing a physical exam, and running lab tests. This helps us pick the right patients for CAR T-cell therapy.
Pre-treatment Preparation
After deciding a patient is eligible, we start getting them ready for treatment. This includes lymphodepletion, which lowers lymphocyte numbers. This makes the body ready for the CAR T cells.
Patients also get a full medical check to spot any risks. This might include heart checks, tests for infections, and brain tests. We make sure they’re ready for the treatment.
Post-treatment Monitoring
After the treatment, patients need to be watched for side effects like cytokine release syndrome (CRS) and brain problems. We keep a close eye on them and deal with any issues fast.
We also check how well the treatment is working. We adjust their care as needed to get the best results. This ongoing care is vital for the success of CAR T-cell therapy.
The Global Research Landscape for CAR T Therapies
The field of CAR T cell therapy is growing fast, with research happening all over the world. This global effort is key to making CAR T therapies better. It shows how important the world’s research is for this new treatment.
Major Research Centers and Initiatives
Top research centers are leading in CAR T cell therapy. Places like the National Cancer Institute, Memorial Sloan Kettering Cancer Center, and the University of Pennsylvania’s Abramson Cancer Center are doing a lot of work. They are learning more about CAR T therapies and making them safer and more effective.
Notable Research Initiatives:
- Investigations into next-generation CAR T cell designs
- Studies on the application of CAR T therapy in solid tumors
- Research on combination therapies to enhance CAR T cell efficacy
Emerging International Developments
Research on CAR T cell therapy is happening worldwide. Countries like China, the United Kingdom, and Australia are making big contributions. They are bringing new ideas and improvements to the field.
Key international developments include:
- China’s rapid advancement in CAR T therapy clinical trials
- The UK’s focus on developing off-the-shelf CAR T products
- Australia’s research into CAR T therapy for rare cancers
Collaborative Clinical Trials
Working together on clinical trials is key to improving CAR T therapies. Research centers and companies can share knowledge and resources. This helps make treatments better for patients.
Examples of collaborative efforts include:
- Multi-center trials evaluating the safety and efficacy of new CAR T products
- Industry-academia collaborations to develop novel CAR T cell technologies
- Global partnerships to expand access to CAR T therapies in diverse patient populations
As research on CAR T therapies grows, we’ll see big improvements soon. The work of major centers, new international efforts, and joint trials will shape the future of CAR T cell therapy.
Conclusion: Transforming Cancer Care Through Cellular Engineering
CAR T-cell therapy is changing how we fight cancer. It uses cellular engineering to find and kill cancer cells. This new way of treating cancer has brought hope to many patients.
These CAR T cells are made to spot and attack cancer. They offer a treatment that’s tailored to each patient. In blood cancers, CAR T-cell therapy has shown great success, with many patients going into remission.
The future of CAR T cell therapy looks promising. Researchers are working to use it for solid tumors too. They also want to make these treatments safer and more effective. As we keep improving, we’ll see even more ways to fight cancer.
The outlook for CAR T cell therapy is very good. We’re looking at new CAR designs and products that can be used by more people. With ongoing research, we’re dedicated to providing top-notch healthcare and support to patients worldwide.
What are CAR T cells and how do they work?
CAR T cells are a new way to fight cancer. They are made by changing a patient’s T cells to find and kill cancer cells. This is done by adding a special receptor that lets the cells see cancer.
How is CAR T-cell therapy different from traditional cancer treatments?
CAR T-cell therapy is a big change in how we treat cancer. It uses the patient’s own cells to fight cancer. This is more targeted and might work better than old treatments.
What is the process of manufacturing CAR T cells for therapy?
Making CAR T cells involves a few steps. First, we take the patient’s T cells. Then, we change them to have the special receptor. After that, we grow more of these cells and put them back in the patient. This takes a few weeks and needs special places and people.
What are the approved indications for CAR T-cell therapy?
CAR T-cell therapy is approved for some blood cancers. This includes leukemia, lymphoma, and multiple myeloma. The exact uses depend on the product and the patient’s situation.
What are the possible side effects of CAR T-cell therapy?
Side effects can include problems with the immune system and the brain. We use medicine and watch patients closely to manage these issues. It’s important to talk to a doctor about the risks and benefits.
How effective is CAR T-cell therapy in treating blood cancers?
CAR T-cell therapy has shown great results in some blood cancers. It has helped many patients get better. But, how well it works depends on the cancer type and the patient’s health.
What are the challenges in using CAR T-cell therapy to treat solid tumors?
Using CAR T-cell therapy for solid tumors is harder. The tumor environment and choosing the right target are big challenges. Researchers are working to solve these problems to help more patients.
What is the cost of CAR T-cell therapy, and is it covered by insurance?
CAR T-cell therapy is expensive. Insurance coverage varies. It’s best to talk to a doctor or financial advisor about the cost and what insurance covers.
What is the future of CAR T-cell technology?
The future of CAR T-cell therapy looks bright. Researchers are working on new versions, products that don’t need to be made for each patient, and treating more types of cancer. These changes aim to make the therapy better and more available.
How can patients access CAR T-cell therapy?
Patients can get CAR T-cell therapy at cancer centers that offer it. Each center has its own rules for who can get the treatment. It’s best to talk to a doctor to find the right place.
What is the role of gene therapy in CAR T-cell therapy?
Gene therapy is key in CAR T-cell therapy. It lets us change T cells to find and kill cancer. This makes the therapy more effective.
How does CAR T-cell therapy relate to adoptive cell therapy?
CAR T-cell therapy is a part of adoptive cell therapy. This approach uses a patient’s own cells to fight cancer. It includes CAR T-cell therapy and other ways to use the immune system against cancer.
What are the possible benefits of off-the-shelf CAR T products?
Off-the-shelf CAR T products could make therapy more available and faster. This could help more patients and make the treatment more effective.