Cancer involves abnormal cells growing uncontrollably, invading nearby tissues, and spreading to other parts of the body through metastasis.
Send us all your questions or requests, and our expert team will assist you.
Gastric cancer is often called “the great masquerader” because its early symptoms are mild and easy to miss. At first, the tumor is small and stays in the inner stomach layers, so it usually does not affect digestion. People may have symptoms similar to common problems like indigestion, gastritis, or ulcers. This makes it easy to mistake for something less serious, leading to delays in diagnosis. Many people use antacids or acid blockers for months, feeling better for a while as the cancer continues to grow.
The most common early symptom is a vague discomfort in the upper belly, called the epigastrium. This can feel like burning, mild nausea, or heaviness. Unlike the sharp pain of an ulcer, cancer pain is usually dull and steady. Another early sign is feeling full quickly, even after eating a small amount. This happens because the stomach wall becomes stiff from the tumor or because a mass takes up space inside the stomach.
Heartburn or acid reflux is also common, especially when the tumor is near the top of the stomach. As the cancer grows, it can affect the valve that keeps acid out of the esophagus. Since acid reflux is common, this symptom is often ignored unless it starts suddenly in an older person or does not get better with usual treatments. Any ongoing change in digestion should be checked by a doctor.
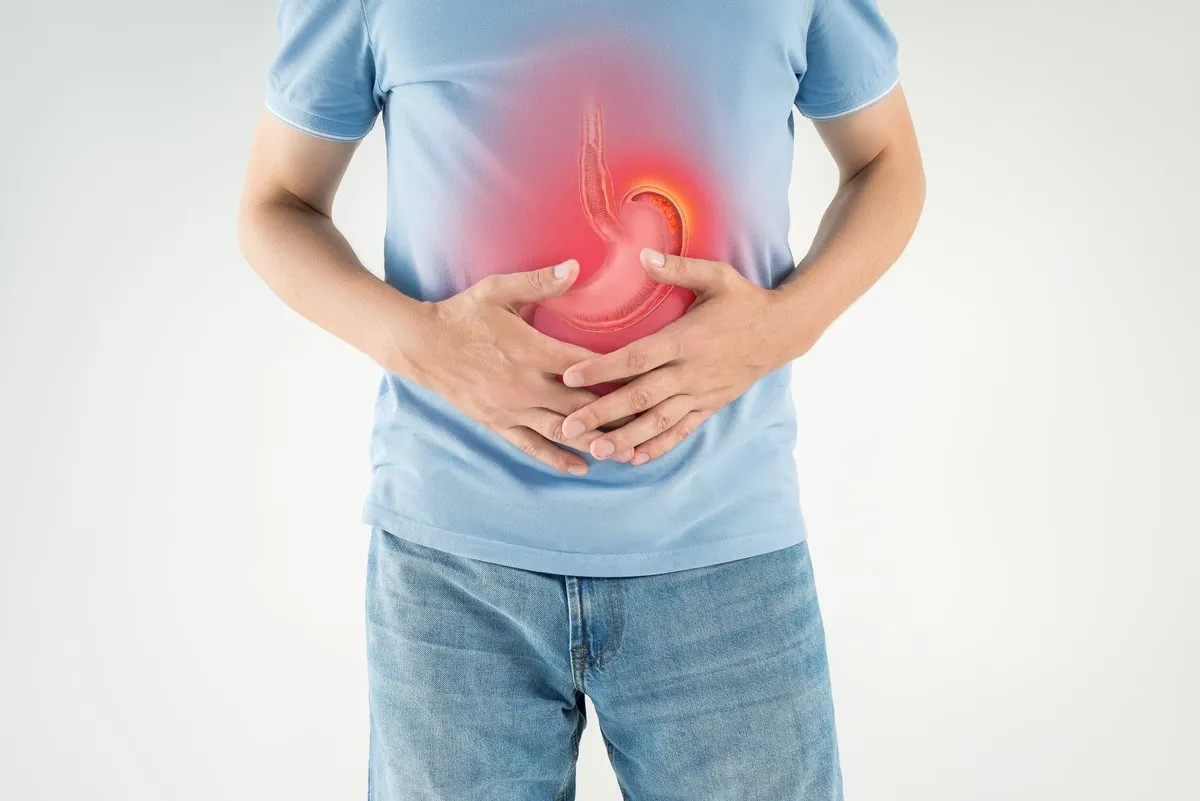
As the cancer advances and penetrates deeper into the stomach wall or invades nearby structures, the symptoms become more alarming and physiologically disruptive. Dysphagia, or difficulty swallowing, is a hallmark of tumors located at the gastroesophageal junction. Patients describe a sensation of food sticking in the chest, initially with solid foods such as meat and bread, and eventually with liquids. This mechanical obstruction leads to rapid nutritional decline.
Unintentional weight loss is one of the most significant prognostic indicators. This weight loss is multifactorial: it results from reduced caloric intake due to obstruction or early satiety, as well as from the tumor’s metabolic demands. The cancer induces a catabolic state, releasing cytokines that break down muscle and fat stores, a condition known as cancer cachexia. Anemia is another frequent complication, presenting as fatigue, paleness, or shortness of breath. This anemia is usually caused by chronic, microscopic bleeding from the tumor’s ulcerated surface, leading to iron deficiency.
In rare but severe cases, the tumor can obstruct the stomach’s outlet (the pylorus). This causes gastric outlet obstruction, characterized by projectile vomiting of undigested food eaten hours earlier. Because the food cannot pass into the intestine, the patient rapidly becomes dehydrated and malnourished. Additionally, palpable masses in the abdomen or the enlargement of the liver due to metastasis are late signs that indicate a high burden of disease.
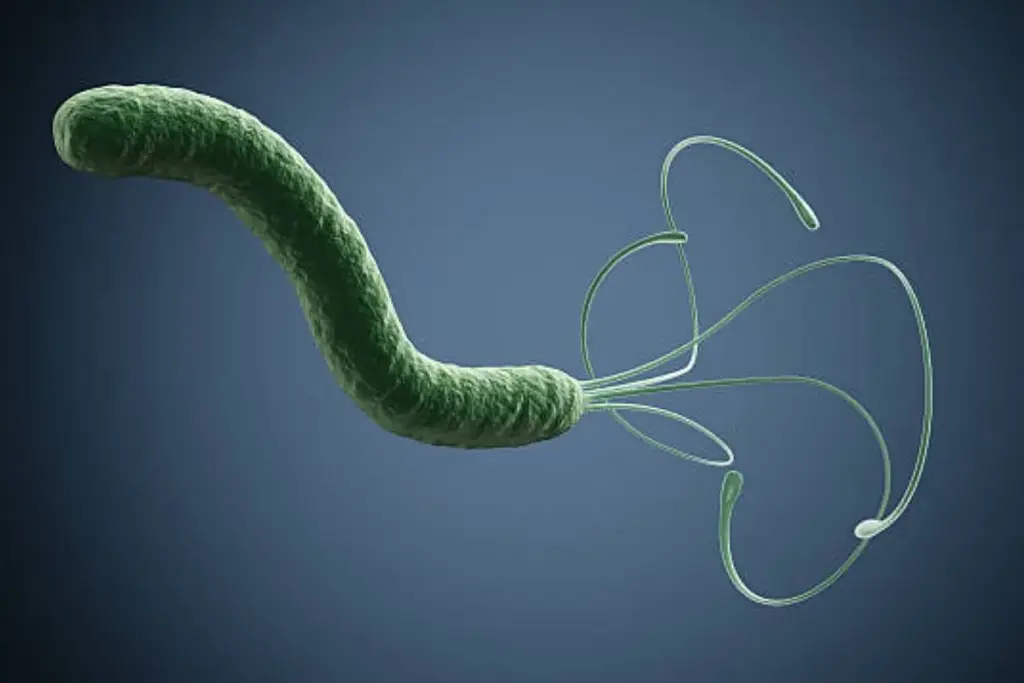
The discovery of Helicobacter pylori and its link to gastric cancer is one of the most significant medical breakthroughs of the 20th century. This spiral-shaped bacterium has evolved to survive in the hostile, acidic environment of the human stomach. It burrows into the mucous layer and secretes an enzyme called urease, which neutralizes the surrounding acid. While half the world’s population harbors this bacterium, only a small fraction develops cancer, indicating a complex interaction between the bacterial strain, the host’s genetics, and the environment.
The mechanism of carcinogenesis involves chronic inflammation. H. pylori injects a toxin called CagA into the stomach cells. This toxin disrupts cellular signaling, causing the cells to lose their shape and divide uncontrollably. Over decades, this constant inflammatory assault triggers the cascade of atrophic gastritis, metaplasia, and dysplasia. The body’s immune response, attempting to clear the infection, releases free radicals that further damage the DNA of the stomach lining cells.
Eradicating H. pylori with antibiotics is the most effective strategy for preventing gastric cancer. However, there is a “point of no return.” If the stomach lining has already undergone extensive intestinal metaplasia (permanent changes), clearing the bacteria may reduce the risk, but it does not eliminate it. This underscores the importance of screening and treating the infection in young adulthood before irreversible damage to the gastric mucosa occurs.
While infection provides the spark, diet often provides the fuel for gastric cancer. Historically, the preservation of food through salting, pickling, and smoking was essential for survival, but these methods are strongly linked to gastric malignancy. High salt intake damages the protective mucosal lining of the stomach, making it more susceptible to carcinogens and facilitating H. pylori colonization. Furthermore, nitrates and nitrites found in processed meats are converted in the stomach into N-nitroso compounds, potent mutagens that can cause direct DNA damage.
Conversely, a diet rich in fresh fruits and vegetables is protective. These foods contain antioxidants, such as Vitamin C, which can inhibit the formation of N-nitroso compounds and scavenge free radicals. The decline in gastric cancer in the West is partly attributed to the widespread use of refrigeration, which enables the year-round consumption of fresh produce and reduces reliance on salt-preserved foods.
Tobacco smoking is another major, modifiable risk factor. Tobacco smoke contains countless carcinogens that are swallowed and bathe the gastric mucosa. Smokers have a significantly higher risk of developing tumors in the proximal stomach and near the esophagus. Alcohol consumption is also a risk factor, particularly when combined with smoking, as alcohol acts as a solvent, potentially enhancing the penetration of tobacco carcinogens into the lining cells. Obesity, specifically central adiposity, increases the risk of cardia gastric cancer by promoting chronic inflammation and gastroesophageal reflux.

Although most cases are sporadic, genetics plays a critical role in susceptibility. The most well-defined hereditary condition is Hereditary Diffuse Gastric Cancer (HDGC), caused by a germline mutation in the CDH1 gene. This gene codes for E-cadherin, a protein responsible for cell-to-cell adhesion. Individuals with this mutation have a lifetime risk of gastric cancer that can exceed seventy percent. Because the tumor is diffuse and starts in the deep layers, it is often invisible on endoscopy. Therefore, prophylactic total gastrectomy (removal of the stomach) is usually recommended for carriers in their early twenties.
Other genetic syndromes also carry an increased risk. Lynch Syndrome (HNPCC), primarily known for colon cancer, also predisposes individuals to gastric cancer, typically the intestinal type. Familial Adenomatous Polyposis (FAP), Li-Fraumeni Syndrome, and Peutz-Jeghers Syndrome are other conditions where gastric surveillance is part of the management protocol.
Host factors such as blood type also play a curious role; individuals with Type A blood have a consistently observed, roughly twenty percent higher risk of developing stomach cancer compared to other blood groups. The reason remains a subject of research but may relate to changes in mucous secretion or susceptibility to H. pylori. Additionally, a history of previous stomach surgery (such as for ulcers) increases risk significantly after 15-20 years, as the altered anatomy allows bile to reflux into the stomach, causing chronic chemical irritation.


Send us all your questions or requests, and our expert team will assist you.
Salt is not a carcinogen itself, but in high concentrations, it acts as an abrasive irritant. It strips away the stomach’s protective mucus layer, exposing the cells to acid and other toxins. It also causes cells to divide more rapidly to repair the damage, increasing the risk of genetic errors, and enhancing H. pylori’s ability to colonize the stomach.
Dyspepsia (indigestion) is usually intermittent, meal-related, and often relieved by antacids or belching. Cancer pain tends to be more constant, progressive (getting worse over time), and may not be fully relieved by medication. However, the overlap is significant, which is why any “indigestion” lasting more than two weeks requires medical evaluation.
Directly, no. There is no evidence that psychological stress causes cancer. However, stress can lead to behaviors like smoking, excessive alcohol consumption, or poor diet, which are risk factors. Stress can also exacerbate symptoms of gastritis or ulcers, which might mimic or mask the symptoms of cancer.
Yes, H. pylori is contagious. It is thought to spread from person to person through saliva (kissing) or through fecal-oral contamination (poor hygiene or contaminated water). Most infections are acquired in childhood. However, having an infected partner does not guarantee you will get the disease or cancer, as hygiene standards and immune systems vary.
Yes. Pernicious anemia is an autoimmune condition where the body attacks the cells in the stomach that help absorb Vitamin B12. This leads to profound inflammation and atrophy (thinning) of the stomach lining. Patients with pernicious anemia have a significantly higher risk of developing gastric cancer and usually require regular endoscopic surveillance.

Medical imaging tests, like CT scans, use contrast agents to make internal structures clearer. These substances are usually safe but can upset some people’s stomachs.


Can stomach cancer cause itchy skin? Many people are unaware that stomach cancer can present in unusual ways.. One of these is itchy skin. This

Leave your phone number and our medical team will call you back to discuss your healthcare needs and answer all your questions.
Your Comparison List (you must select at least 2 packages)