Orthopedics is one of the most established fields for the application of regenerative medicine.
Send us all your questions or requests, and our expert team will assist you.
Regenerative medicine is often portrayed in the media as a “cure-all” for every human ailment. At Liv Hospital, we believe in Evidence-Based Regeneration. We do not offer stem cell therapy for every condition under the sun. Instead, we focus on specific clinical areas where scientific research and our own clinical experience show a clear benefit.
Stem cells work best in environments where the body is trying to heal but lacks the resources—specifically blood flow and growth factors. They act as “biological contractors,” arriving at the site of damage and coordinating the repair process through Paracrine Signaling (releasing healing proteins) and Immunomodulation (calming inflammation).
This guide outlines the primary conditions we treat, divided into Orthopedics, Aesthetics, Systemic Health, and Wound Care.
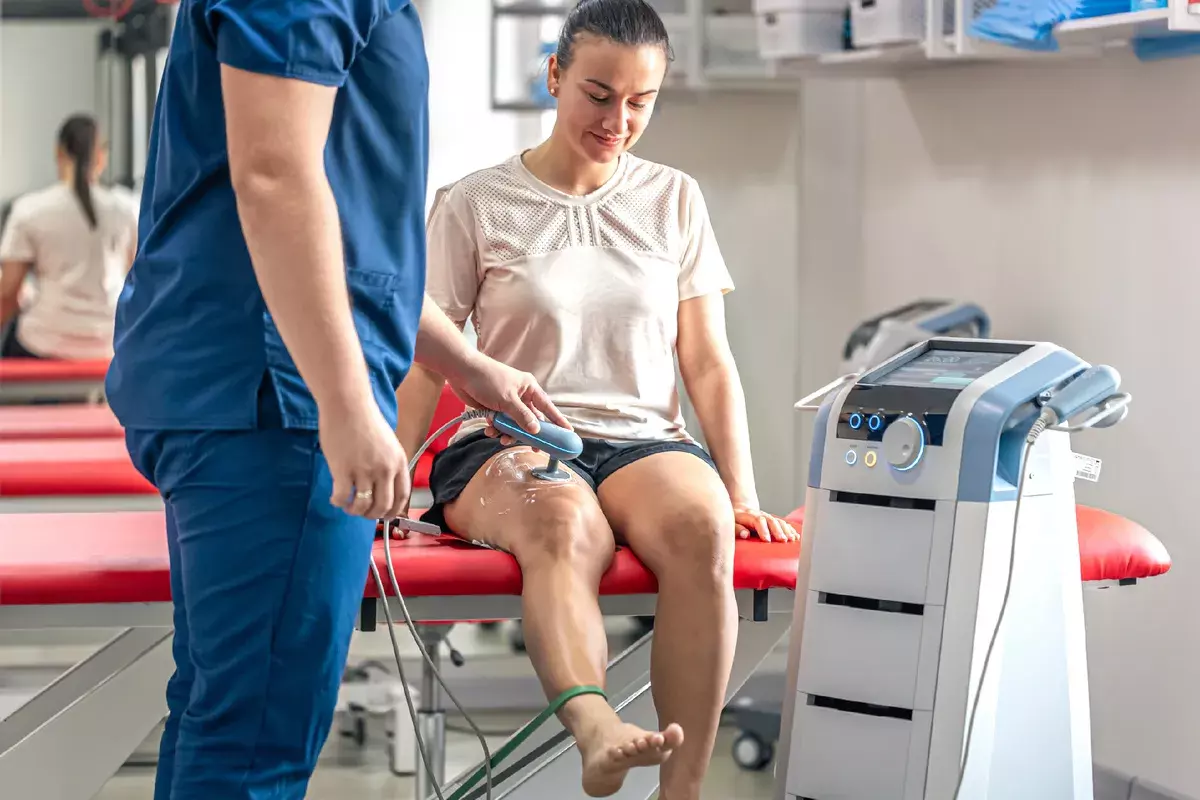
Joints are the perfect candidates for stem cell therapy. Why? Because cartilage and tendons have a very poor blood supply (avascular). When they are injured, they cannot bring in enough repair cells on their own. Injecting stem cells directly into the joint bridges this gap.

Send us all your questions or requests, and our expert team will assist you.
Aging is essentially a slow loss of stem cell activity. The skin thins, fat pads shrink, and blood flow decreases. We use stem cells not just to fill, but to revitalize.
Stem cells are powerful immune regulators. They don’t boost the immune system; they balance it.
This is an area of ongoing research. We approach it with caution and transparency.
To ensure safety and success, we do not perform stem cell therapy on every patient.
Biological therapy is not like a mechanical fix. Not everyone responds the same way.
They can reverse early hair loss. If the hair follicle is still alive but dormant (miniaturized), stem cells can wake it up and make the hair thicker. However, if the area has been completely bald and shiny for 10 years (follicle death), stem cells cannot grow new hair from scratch. In that case, we recommend a Hair Transplant.
It is long-lasting, but rarely “permanent” in the sense of a metal implant. Most patients experience relief for 3–5 years. Because you are still walking on the knee and aging, the wear and tear continues. The procedure can be repeated safely if needed.
Currently, stem cell therapy cannot regenerate the insulin-producing beta cells in the pancreas enough to cure Type 1 Diabetes completely. While research is promising (using islet cell transplants), it is not yet a standard clinical treatment at our center. We focus on Type 2 Diabetes management.
Viagra works chemically for a few hours. Stem cells work biologically to repair the blood vessels. The goal of stem cell therapy is to allow you to have spontaneous erections without needing a pill every time. It offers a functional restoration, not just a temporary fix.
Amyotrophic Lateral Sclerosis (ALS) is a rapidly progressive and fatal disease. While some clinics offer stem cells for ALS, large clinical trials have unfortunately failed to show a consistent benefit in stopping the disease. At Liv Hospital, we adhere to evidence-based medicine and do not offer treatments that give false hope without scientific backing.

Choosing how many embryos to transfer in IVF is key in fertility care.how many embryos are transferred in ivfWhere do embryonic stem cells come from?
Period after ivf egg retrieval, many women wonder when their period will come back. We know this can be a worrying time. It’s important to

Leave your phone number and our medical team will call you back to discuss your healthcare needs and answer all your questions.
Leave your phone number and our medical team will call you back to discuss your healthcare needs and answer all your questions.
Your Comparison List (you must select at least 2 packages)