Cancer involves abnormal cells growing uncontrollably, invading nearby tissues, and spreading to other parts of the body through metastasis.
Send us all your questions or requests, and our expert team will assist you.
The diagnostic pathway for sarcoma is a high-stakes exercise in anatomical precision and biological characterization. Given the rarity of the disease and the potential for benign mimics, misdiagnosis is a frequent and dangerous pitfall. The objective of the diagnostic phase is not merely to confirm the presence of malignancy but to define the tumor’s histological subtype, grade (biological aggressiveness), and exact anatomical relationship to critical neurovascular structures. Staging is the linguistic framework that translates biological data into a prognostic category, guiding crucial decisions between limb-salvage surgery and amputation, or between localized treatment and systemic chemotherapy.
The initial evaluation begins with advanced cross-sectional imaging. For soft tissue masses of the extremities or trunk, Magnetic Resonance Imaging (MRI) is the gold standard. MRI provides exquisite soft-tissue contrast, allowing the radiologist to distinguish the tumor from surrounding muscle, fat, and edema. It is critical to define the “compartment” of the cancer—whether it is contained within a specific muscle group or has breached the fascial barriers. For bone sarcomas, plain radiographs (X-rays) provide the first clue, revealing characteristic patterns of bone destruction (osteolysis) and new bone formation (osteoid). An MRI follows this to assess the extent of the tumor within the marrow space and any soft tissue extension.
The biopsy is the single most critical step in the management of sarcoma. Unlike carcinomas, where excisional biopsy (removing the whole lump) is sometimes appropriate, “shelling out” a sarcoma without knowing the diagnosis is a catastrophic error. It contaminates the surrounding tissue planes with microscopic tumor cells, necessitating much broader radiation fields or even amputation to achieve local control. The standard of care is a core needle biopsy, typically performed under image guidance (ultrasound or CT). This technique provides a cylinder of tissue sufficient for histological analysis of architecture and molecular testing while minimizing the risk of tumor seeding along the needle track. The biopsy track must be carefully planned so that it can be excised en bloc with the tumor during the definitive surgery.

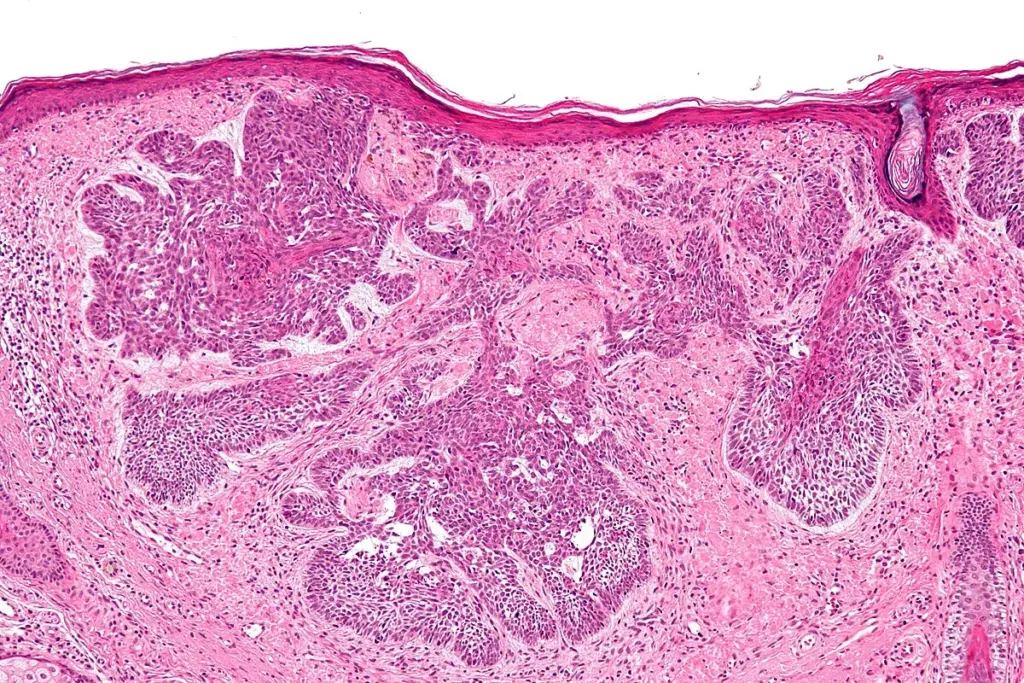
In the modern era, the diagnosis of sarcoma relies heavily on molecular pathology. Because many sarcomas appear similar under the microscope (spindle cells or small, round, blue cells), traditional histology is often insufficient. Fluorescence In Situ Hybridization (FISH) and Reverse Transcription Polymerase Chain Reaction (RT-PCR) are used to detect the specific chromosomal translocations that define many sarcoma subtypes. For example, detecting the EWSR1 gene rearrangement confirms Ewing Sarcoma, while the SYT-SSX fusion confirms Synovial Sarcoma. Next-Generation Sequencing (NGS) panels are increasingly used to identify rare mutations or fusion partners in “undifferentiated” sarcomas, potentially revealing targets for personalized therapy.
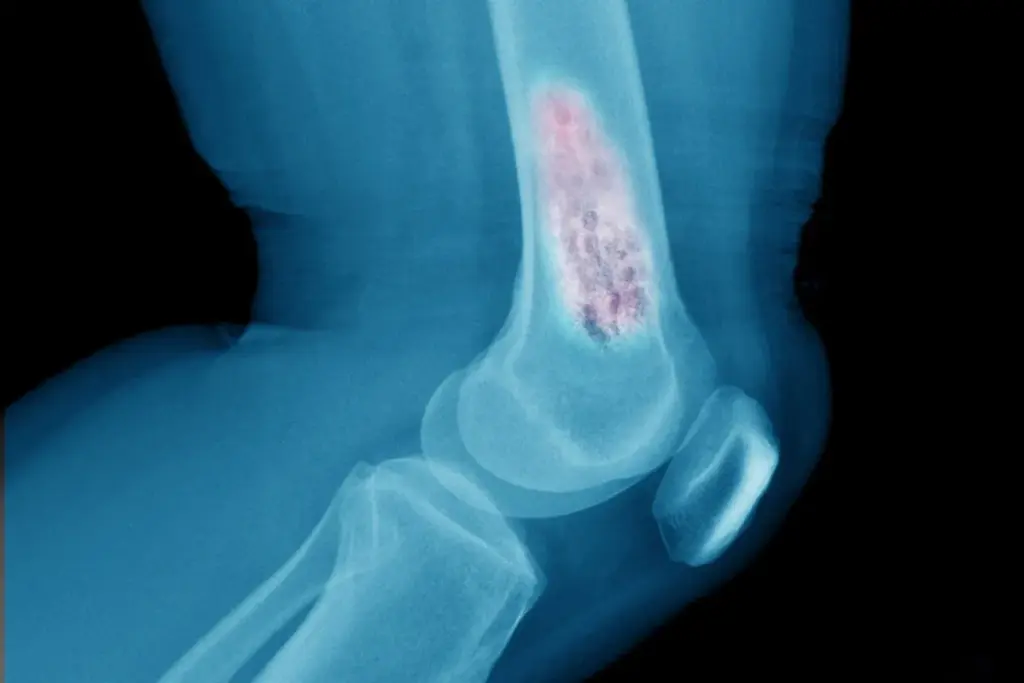
Sarcoma staging follows the TNM (Tumor, Node, Metastasis) system but places a unique and significant emphasis on Grade (G). The histological grade—determined by the degree of cellular differentiation, mitotic rate (speed of division), and the amount of tumor necrosis—is the most powerful predictor of metastasis and survival.
Unlike in carcinomas, lymph node involvement is rare in most sarcomas (except for specific subtypes such as Epithelioid Sarcoma, Rhabdomyosarcoma, and Clear Cell Sarcoma). Hence, the “N” stage is often negative even in advanced disease. Instead, hematogenous spread to the lungs is the primary concern for metastasis.
A significant challenge in sarcoma diagnosis is ruling out benign mimics. A rapidly growing mass could be a hematoma (blood collection), an abscess, or Myositis Ossificans (heterotopic bone formation in muscle after trauma). Benign tumors like lipomas, schwannomas, and desmoid tumors can be radiologically indistinguishable from low-grade malignancies. Desmoid tumors (aggressive fibromatosis), while technically benign because they do not metastasize, are locally invasive and infiltrate muscle planes like a malignancy, requiring similar diagnostic rigor. The distinction between a benign “Atypical Lipomatous Tumor” and a malignant “Well-Differentiated Liposarcoma” often relies on the anatomical location (extremity vs. retroperitoneum) and MDM2 gene amplification status.
A significant challenge in sarcoma diagnosis is ruling out benign mimics. A rapidly growing mass could be a hematoma (blood collection), an abscess, or Myositis Ossificans (heterotopic bone formation in muscle after trauma). Benign tumors like lipomas, schwannomas, and desmoid tumors can be radiologically indistinguishable from low-grade malignancies. Desmoid tumors (aggressive fibromatosis), while technically benign because they do not metastasize, are locally invasive and infiltrate muscle planes like a malignancy, requiring similar diagnostic rigor. The distinction between a benign “Atypical Lipomatous Tumor” and a malignant “Well-Differentiated Liposarcoma” often relies on the anatomical location (extremity vs. retroperitoneum) and MDM2 gene amplification status.
Before initiating treatment, a comprehensive systemic evaluation is required. Echocardiography is performed to assess cardiac function before the use of anthracyclines (such as Doxorubicin), a cornerstone of sarcoma chemotherapy known for its cardiotoxicity. Audiometry is necessary if cisplatin is considered (which is common in osteosarcoma). Fertility counseling is paramount for pediatric and young adult patients, as chemotherapy can result in sterility. Functional assessments, including range-of-motion and gait analysis, establish a baseline for post-surgical rehabilitation.

Send us all your questions or requests, and our expert team will assist you.
An excisional biopsy removes the entire lump, which is only appropriate for tiny, superficial masses (under 3cm) suspected to be benign. An incisional biopsy (or core needle biopsy) takes only a sample of the tumor. For potential sarcomas, core biopsy is preferred because removing the whole tumor without proper margins can spread cancer cells into the surrounding tissue, complicating future surgery.
Sarcomas have a strong tendency to spread through the bloodstream (hematogenous metastasis), and the lungs are the most common site for these secondary tumors to land. A chest CT is the most sensitive way to detect small lung nodules (metastases) that would not be visible on a regular X-ray, which is critical for accurate staging.
The “grade” refers to how aggressive the cancer cells look under a microscope. Grade 1 (low-grade) tumors look more like normal cells and grow slowly. Grade 3 (high-grade) tumors look very abnormal, divide rapidly, and have areas of dead tissue (necrosis). High-grade tumors are much more likely to spread and usually require more aggressive treatment like chemotherapy.
Most sarcomas typically do not spread to lymph nodes; they spread through the blood to the lungs. However, there are exceptions. Specific subtypes like Rhabdomyosarcoma, Epithelioid Sarcoma, Clear Cell Sarcoma, and Angiosarcoma tend to spread to lymph nodes, so doctors check nodes more carefully in these diagnoses.
Muscles in the arms and legs are grouped into “compartments” separated by rigid fibrous sheets called fascia. Sarcomas tend to stay contained within these fascial boundaries for a long time. A compartment resection is a surgical procedure that removes the tumor along with all the muscles and tissues within that specific compartment to ensure no cancer cells are left behind.


Advanced skeletal imaging techniques can show detailed insights into your bone health. They can detect conditions such as fractures, bone metastases, infections, and degenerative bone
Finding bone cancer needs a detailed approach. At Liv Hospital, we use the latest imaging tech for accurate diagnosis and treatment plans. The right scan
A diagnosis of a brain tumor can be scary. But new medical tech has brought non-surgical treatments that change lives. Would a PET scan show
Finding bone cancer early is key to good treatment. Tests like PET scans, MRI, CT scans, and blood tests are important. They help us see
PET Scan and Bone Cancer: Early Detection for Better Care Finding bone cancer early is vital for effective treatment and recovery. At Liv Hospital, our

Leave your phone number and our medical team will call you back to discuss your healthcare needs and answer all your questions.
Your Comparison List (you must select at least 2 packages)