Cancer involves abnormal cells growing uncontrollably, invading nearby tissues, and spreading to other parts of the body through metastasis.
Send us all your questions or requests, and our expert team will assist you.
Sarcoma often develops quietly, which means it is usually diagnosed late, when the disease is already advanced. Unlike other cancers that cause early symptoms like bleeding or blockage, sarcomas grow deep in the body’s connective tissues, such as the abdomen, muscles, or bones. Symptoms usually appear only when the tumor gets big enough to push aside normal tissues. The causes of sarcoma are complex and can include random genetic changes, inherited risks, and certain environmental exposures. Recognizing symptoms can be difficult because it depends on how the tumor affects the body’s structure and space.
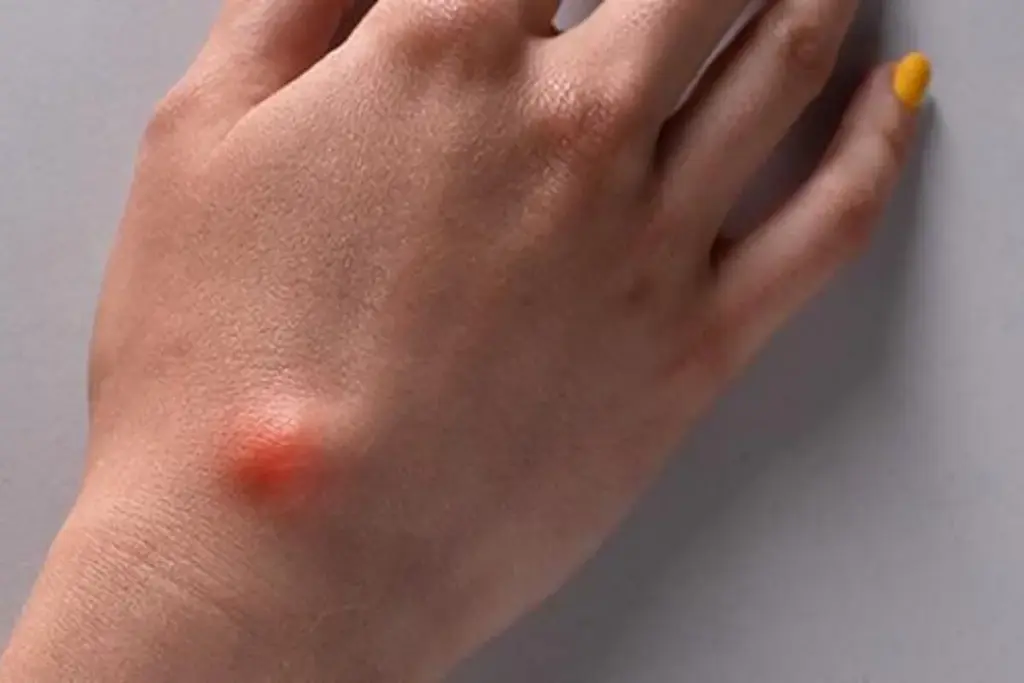
The most common sign of a soft tissue sarcoma is a painless lump that slowly gets bigger. Because it doesn’t hurt at first, people often think it’s just a harmless lump, bruise, or sports injury. Pain usually happens later, when the tumor presses on nerves or stretches tissues. Bone sarcomas, like osteosarcoma, are different—they often cause a deep, aching pain that isn’t related to movement and gets worse at night. This pain is caused by pressure inside the bone and tiny fractures as the tumor grows.
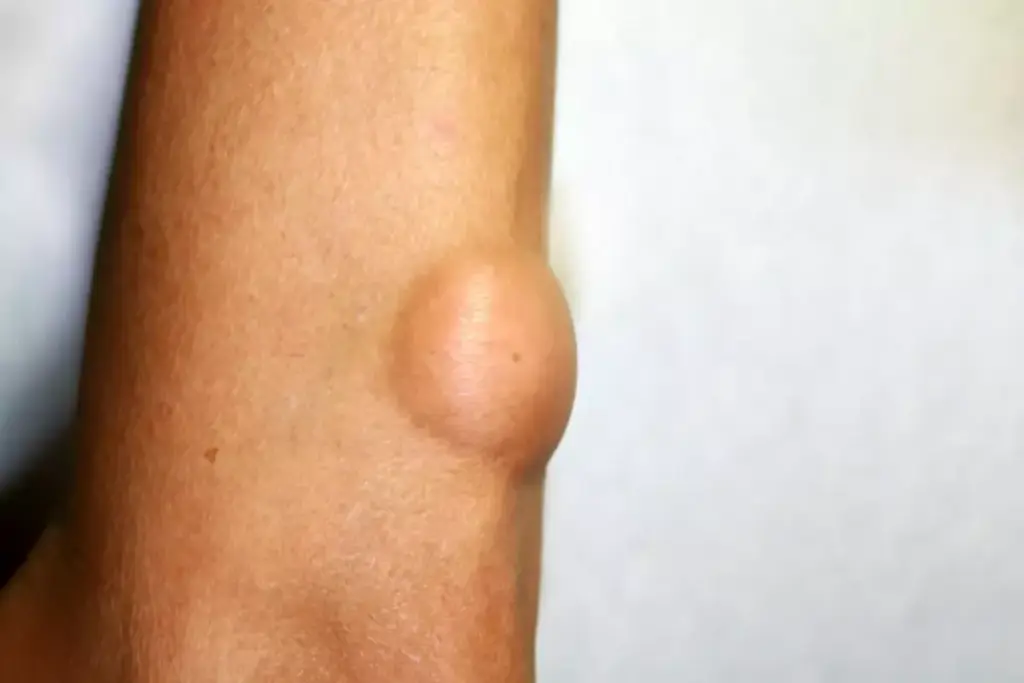
As a sarcoma expands, it exerts a “mass effect” on the surrounding structures. In the extremities, this can manifest as distal edema (swelling) due to the compression of the deep venous system or lymphatic channels. A sarcoma arising in the popliteal fossa (behind the knee) or the femoral triangle may mimic a deep vein thrombosis. Neurovascular compromise is another hallmark; compression of the sciatic or femoral nerve can lead to radicular pain, paresthesia (numbness), or motor weakness in the distribution of the affected nerve. This neuropathic pain is distinct from the somatic pain of the tumor itself and indicates local invasion or entrapment.
In the retroperitoneum and abdomen, sarcomas can grow to enormous dimensions—often exceeding 20 centimeters—before causing symptoms. The abdominal cavity accommodates this slow expansion until the tumor begins to obstruct the gastrointestinal tract, causing early satiety, nausea, or constipation. Ureteral obstruction can lead to hydronephrosis and flank pain. These tumors, such as liposarcomas or leiomyosarcomas, essentially hijack the potential space of the abdomen, growing silently until they compromise vital organ function through sheer physical displacement.
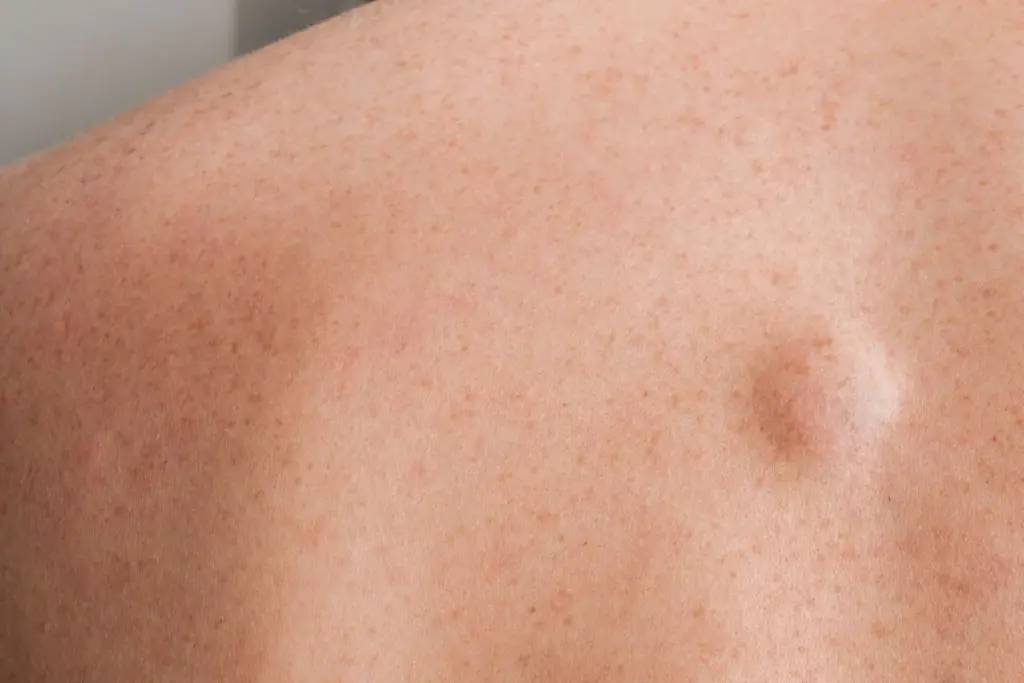
The sensation of pain in sarcoma is not merely mechanical but is mediated by a complex molecular cross-talk between the tumor cells and the peripheral nervous system. The sarcoma microenvironment is rich in inflammatory cytokines and neurotrophic factors that sensitize local nociceptors (pain receptors).
While the majority of sarcomas are sporadic, a significant subset arises in the context of hereditary cancer predisposition syndromes. These syndromes provide a window into the molecular “first hits” required for sarcomagenesis. The most renowned is Li-Fraumeni Syndrome, caused by a germline mutation in the TP53 tumor suppressor gene. Individuals with this syndrome have a catastrophically high risk of developing both soft tissue and bone sarcomas at a young age because their cells lack the fundamental “brake” on cell division and the ability to initiate apoptosis in response to DNA damage.
Neurofibromatosis Type 1 (NF1) is another critical syndrome, caused by mutations in the neurofibromin gene, a negative regulator of the RAS signaling pathway. Patients with NF1 develop multiple benign neurofibromas, which have the potential to transform into Malignant Peripheral Nerve Sheath Tumors (MPNST), a highly aggressive form of sarcoma. This transformation is driven by the accumulation of additional genetic hits, often involving the CDKN2A locus. Retinoblastoma survivors, who carry a germline RB1 mutation, are at a significantly elevated risk for developing osteosarcoma later in life, particularly within the radiation field if they received radiotherapy for their ocular tumor.
Sarcoma is one of the few cancers with a clearly defined link to ionizing radiation. Post-radiation sarcoma is a distinct clinical entity that arises within the treatment field of a previous malignancy, typically with a latency period of 5 to 15 years. The mechanism involves the induction of double-strand DNA breaks and genomic instability in the mesenchymal cells within the radiation field. This is a cruel paradox of modern oncology, where the curative treatment for a carcinoma (such as breast cancer or lymphoma) can seed the ground for a subsequent sarcoma (such as angiosarcoma or undifferentiated pleomorphic sarcoma).
Chemical carcinogenesis also plays a role, albeit less prominently than in carcinomas. Historical exposure to Thorotrast (a radioactive contrast agent), vinyl chloride monomer (used in plastics manufacturing), and arsenic (found in some herbicides and contaminated groundwater) is causally linked to the development of hepatic angiosarcoma. These agents act as direct mutagens, forming DNA adducts that disrupt the fidelity of replication in endothelial cells.
Chronic inflammation and lymphatic stasis promote a pro-tumorigenic microenvironment. Stewart-Treves Syndrome is a specific form of lymphangiosarcoma that arises in the setting of chronic, long-standing lymphedema, most famously in the arm following radical mastectomy for breast cancer. The protein-rich interstitial fluid in lymphedema initiates a chronic inflammatory response, stimulating the proliferation of lymphatic endothelial cells and fibroblasts. Over decades, this unregulated proliferation, combined with a localized failure of immune surveillance (due to lymphatic obstruction), facilitates malignant transformation. This highlights the intricate link between the immune system, the interstitial fluid dynamics, and mesenchymal stability.
Viral etiologies are rare in sarcoma but critically crucial in specific subtypes. Human Herpesvirus 8 (HHV-8), also known as Kaposi Sarcoma-associated Herpesvirus (KSHV), is the causative agent of Kaposi Sarcoma. This virus infects endothelial cells and expresses viral oncogenes (such as v-GPCR and v-Cyclin) that drive cell proliferation, angiogenesis, and the inhibition of apoptosis. The virus hijacks the host cell’s machinery to create a highly vascular, inflammatory tumor. This malignancy is particularly aggressive in the context of immunosuppression, such as in HIV/AIDS or organ transplantation, demonstrating the reliance of the virus on a compromised host immune system to drive tumorigenesis.

Send us all your questions or requests, and our expert team will assist you.
Most soft tissue lumps are benign lipomas (fatty tumors) or cysts. A sarcoma is more likely if the mass is larger than 5 centimeters (about the size of a golf ball), is deep within the muscle rather than just under the skin, is firm to the touch, and is growing in size. Pain is not a reliable differentiator, as many sarcomas are painless in the early stages.
The pain from bone cancer often worsens at night due to a decrease in the body’s natural anti-inflammatory cortisol levels during sleep, as well as the lack of other sensory distractions. Furthermore, the tumor exerts pressure on the rigid bone and causes microfractures in the weakened cortex, generating a deep, gnawing ache that is not relieved by resting the limb.
The vast majority of sarcomas are sporadic, meaning they occur by chance. However, specific genetic syndromes like Li-Fraumeni Syndrome, Neurofibromatosis Type 1, and Retinoblastoma carry a high risk of sarcoma. A family history of sarcoma, especially in young relatives, or a history of multiple cancers in one person, may prompt genetic testing.
Current medical evidence suggests that a single traumatic injury does not cause sarcoma. However, an injury often draws attention to a pre-existing mass that was previously unnoticed. Patients frequently recall a blow or strain to the area where a sarcoma is later diagnosed, but the trauma was likely the event that led to the discovery, not the cause.
Stewart-Treves Syndrome is a rare, aggressive angiosarcoma that develops in long-standing chronic lymphedema (swelling). It most classically occurs in the upper arm of women who underwent radical mastectomy and lymph node dissection for breast cancer years or decades prior. The chronic swelling and inflammation promote the malignant transformation of lymphatic vessels.

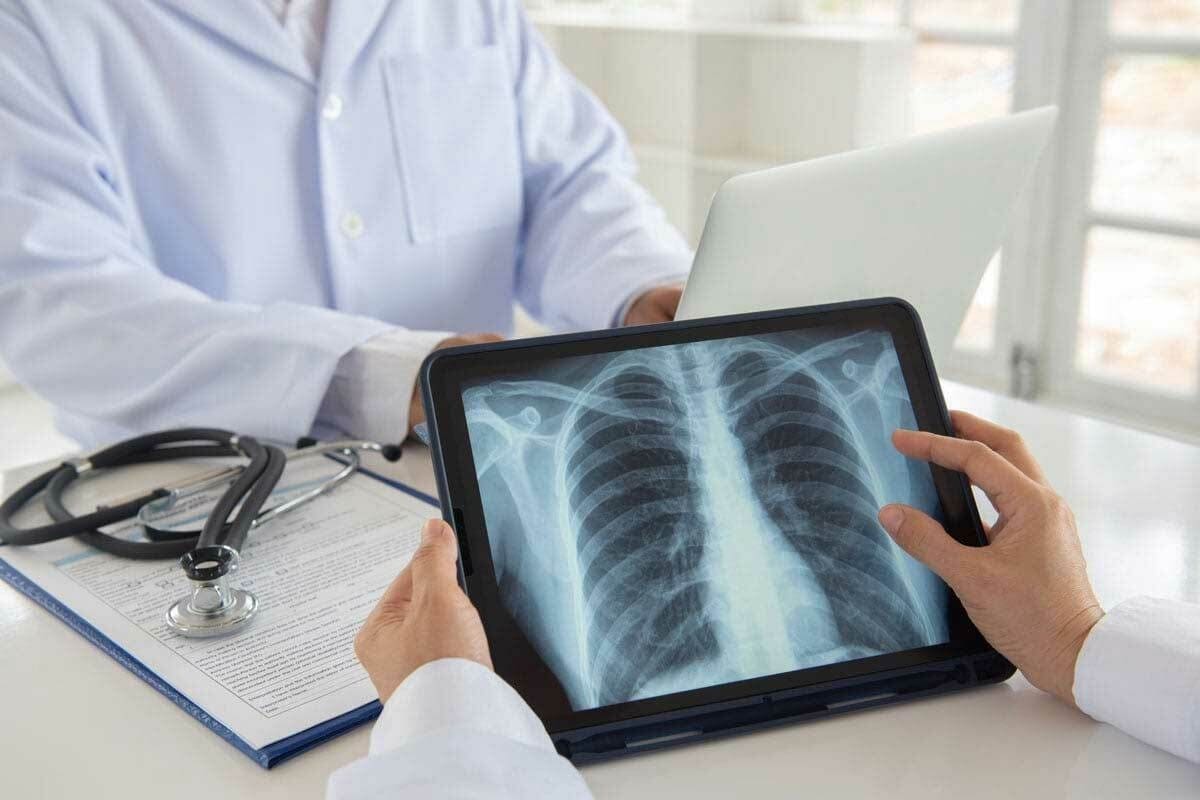
PET Scan and Bone Cancer: Early Detection for Better Care Finding bone cancer early is vital for effective treatment and recovery. At Liv Hospital, our
Knowing how to check for bone cancer is key to early treatment. At Liv Hospital, we use the latest medical methods and care to diagnose
A diagnosis of a brain tumor can be scary. But new medical tech has brought non-surgical treatments that change lives. Would a PET scan show
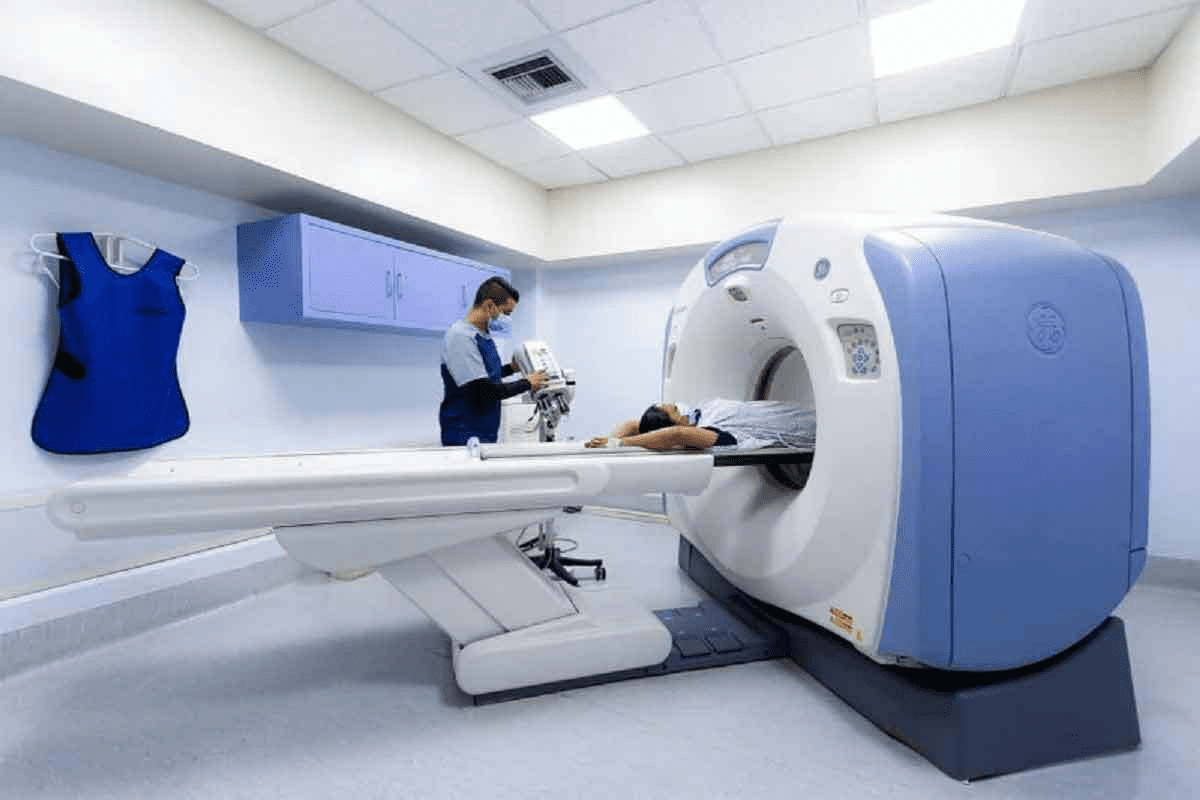
Advanced skeletal imaging techniques can show detailed insights into your bone health. They can detect conditions such as fractures, bone metastases, infections, and degenerative bone
Nearly 1 in 4 cancer patients will see their cancer spread to other parts of the body. Bone is a common place for this to
Finding bone cancer early can really help patients. At Liv Hospital, we use the latest tech and care to help patients. Many tests help find

Leave your phone number and our medical team will call you back to discuss your healthcare needs and answer all your questions.
Your Comparison List (you must select at least 2 packages)